▪
Introduction and Background: Considerations for Using MRI
Magnetic resonance imaging (MRI) is an important tool in imaging the mediastinum, chest wall, and chest vasculature, with a continuously expanding role. Excellent soft tissue contrast makes MRI a useful tool in the diagnosis, staging, surgical planning, and response to treatment evaluation in many cancers. MRI offers sensitivity to blood flow without ionizing radiation, which is particularly useful for multiphase vascular imaging. Multiplanar imaging capabilities allow the precise evaluation of complex thoracic lesion configuration and margins when chest wall, mediastinal, or spinal invasion is suspected.
Although computed tomography (CT) is often the primary means of imaging the chest, the role of MRI is increasing. The indications and application of thoracic MRI and magnetic resonance angiography (MRA) will be reviewed in this chapter while highlighting the MRI appearance of common thoracic pathology.
Recent Technologic Advances That Make Chest MRI More Feasible
Clinically available MRI sequences and those that are frequently used in lung MRI research are summarized in Table 8.1 . Magnetic resonance has shown an increasing role in the imaging of chest disease due to faster sequences with shorter breath-hold times and more robust cardiac gating. The availability of multichannel phased array MRI coils and improvements in parallel imaging are partially responsible for the significant improvements in scan times. In addition, newer gadolinium contrast agents, such as the macrocyclic agents, have decreased the risk of nephrogenic systemic fibrosis.
| SEQUENCE | UTILITY | COMMENTS |
|---|---|---|
| Commercially Available | ||
| T1 | Mass evaluation | Pre- and postcontrast |
| T2 | Mass evaluation, radiation fibrosis vs. recurrence | |
| DCE T1-weighted perfusion | Pulmonary hypertension, chronic obstructive disease, pulmonary embolism | 3D full-chest coverage |
| ASL perfusion | Pulmonary hypertension, chronic obstructive disease | No IV contrast required; better absolute quantitation than DCE 2D evaluation of limited number of slices |
| Diffusion-weighted imaging | Nodule or mass evaluation | |
| Research Tools | ||
| Hyperpolarized 3 He, 129 Xe | Ventilation defects in chronic obstructive disease, asthma, bronchospasm | Requires broadband-capable hardware, polarizer, and specialized physics support |
| Ultrashort TE | Lung cancer, diffuse lung disease, pulmonary edema, air trapping | Not yet available commercially |
Newer proton-based sequences, including ultrashort echo times (TEs) and pulmonary perfusion, are improving the ability of MRI in pulmonary parenchymal evaluation using commercial hardware. The use of hyperpolarized gases has also been increasing the capabilities of pulmonary MRI. With these applications, interstitial lung diseases and chronic obstructive pulmonary disease (COPD) can be evaluated with MRI.
With faster imaging, MRA is now becoming more robust in the rapid evaluation of acute and chronic vascular disease, including pulmonary embolism (PE). Compared to computed tomography angiography (CTA), MRA has the benefit of not requiring the use of ionizing radiation and the ability to scan multiple postcontrast phases following a single contrast dose. In addition, noncontrast MRA sequences are improving and can offer diagnostic evaluation of vasculature when contrast is not used.
▪
Lung MRI
Sequences Used to Evaluate the Lung Parenchyma and Lung Masses
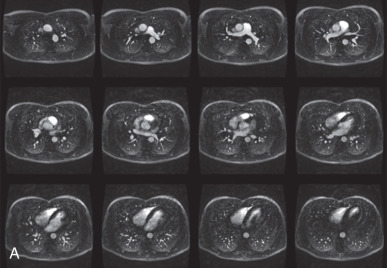
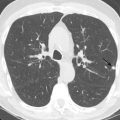
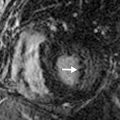
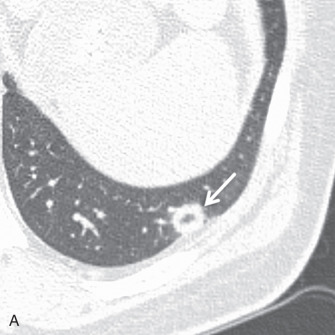
T1 and T2 signal characterization of lung masses ( Fig. 8.1 ) alone has not been shown to be a reliable means of distinguishing between benign and malignant processes. However, more recent studies have suggested restricted diffusion on diffusion-weighted imaging ( Fig. 8.2 ) and offers promise in characterizing benign and malignant lesions.





Three-dimensional (3D) MRI with an ultrashort TE has allowed for high-resolution, whole-lung imaging. Shortening the TE in fast spin-echo sequences is crucial for maintaining image quality in the lungs and has demonstrated clinical utility in assessing non–small cell lung cancer, diffuse lung disease, and pulmonary edema.
Pulmonary imaging with hyperpolarized agents, such as helium-3 ( 3 He) and xenon-129 ( 129 Xe), has allowed functional pulmonary analysis on MRI, which has proven useful in asthma, COPD, and cystic fibrosis. Although the image quality with these agents is high, the need for specialized hardware, technical expertise, and expensive gas has limited the use of these techniques to a few academic centers.
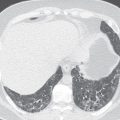
A recent study has shown that 3-T MRI, including T2 half-Fourier acquisition single-shot turbo spin-echo (HASTE) and 3D gradient echo (GRE) sequences, offers sensitivity of 82% and a specificity of 79% in detecting pulmonary abnormalities compared to high-resolution CT (HRCT). MRI was sufficient for detecting infectious nodules and consolidations in neutropenic patients with acute myeloid leukemia; however, MRI failed to detect ground-glass opacities that were noted on HRCT.
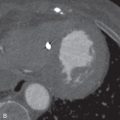
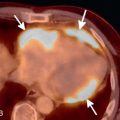
The postcontrast enhancement pattern on MRI has proven more useful for evaluating benign and malignant lesions in the lungs, compared to precontrast T1- and T2-weighted imaging. Malignant pulmonary lesions demonstrate higher maximum enhancement values, with increased slope and earlier peak enhancement, than benign lesions ( Fig. 8.3 ). Delayed enhancement is also higher in malignant compared to benign lesions.

MRI perfusion of the lungs correlates well with pulmonary function tests in COPD patients and can be used to distinguish mild from severe cases of COPD. Pulmonary perfusion data can be acquired using dynamic contrast-enhanced (DCE) or arterial spin labeling (ASL) techniques. With dynamic contrast injection, parenchymal enhancement can be measured precontrast and during multiple postcontrast acquisitions. Lung parenchymal signal intensity is higher with gadobenate dimeglumine (Multihance) compared to gadolinium dimeglumine (Magnevist). There is a nonlinear relationship between contrast agent concentration and MRI signal in the arterial input function, and therefore a nonlinear correction method is useful for perfusion quantification using DCE techniques. ASL is another established technique that can evaluate pulmonary parenchymal perfusion without intravenous (IV) contrast. ASL involves cardiac-gated images obtained during a breath-hold, where the only differing factor between images is the radiofrequency (RF) tagging of the blood flowing into the image. Subtracted images can provide signal proportional to the arterial blood delivered to the lung during a cardiac cycle, which can be quantified. It is, however, more technically challenging than DCE to perform and usually results in only a small number of two-dimensional (2D) images.
When to Use MRI for Characterizing Lung Tumors
Traditionally, pulmonary imaging with MRI has been limited by a paucity of protons in the lung parenchyma, respiratory motion due to longer imaging times, and magnetic field inhomogeneities with air-tissue interfaces in the lungs. In most cases, CT offers better pulmonary imaging than MRI due to its higher spatial resolution and shorter scan times, reducing the possibility of motion artifact.
Solitary Pulmonary Nodules
Solitary pulmonary nodule imaging with MRI has been shown to differentiate lung cancers from tuberculomas and hamartomas reliably using DCE methods and washout ratios. Lung malignancies (see Fig. 8.3 ) have rapid enhancement with mild delayed washout at 8 minutes, whereas tuberculomas and hamartomas have slower steady enhancement over 8 minutes with a lower peak. However, dynamic MRI cannot reliably differentiate between lung cancers and focal organizing pneumonias because both display relatively rapid enhancement, with mild delayed washout. Additional signs that may help differentiate benign and malignant nodules include an enhancing rim at the periphery of a lesion (a thin rim enhancement pattern), which may be a sign of benignity. Similarly, a heterogeneous enhancement pattern with areas of irregular linear enhancement, so-called network enhancement, was more commonly seen in hamartomas than other solitary pulmonary nodules.
Assessing Extrapulmonary Invasion
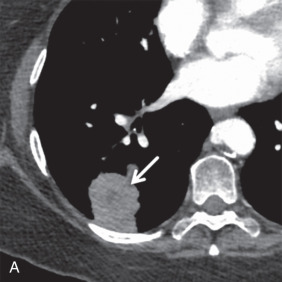
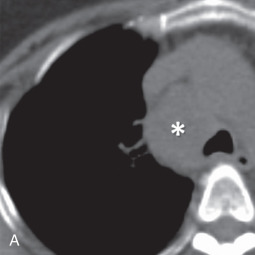
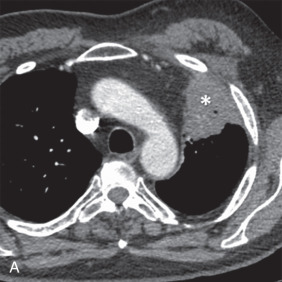
MRI is the primary imaging modality for the local staging of superior sulcus (Pancoast) tumors ( Fig. 8.4 ) due to excellent soft tissue contrast. In addition, MRI can offer definitive evaluation of tumor extension into the great vessels, mediastinum, pericardium, chest wall ( Fig. 8.5 ), and spine in cases where CT is equivocal. MRI is also appropriate for evaluating the pleural involvement of tumors or differentiating pleural metastases from primary tumors of the pleura, such as a solitary fibrous tumor of the pleura ( Fig. 8.6 ). Patients who cannot receive iodinated contrast can undergo MRI, in addition to noncontrast CT, for the evaluation of hilar and mediastinal involvement and lymph node metastases.
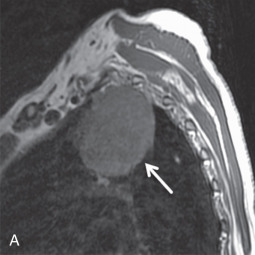





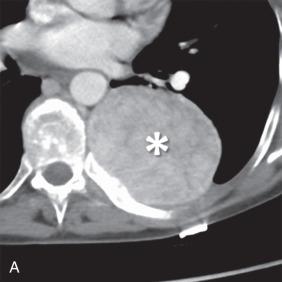



Brachial plexus invasion is well evaluated with MRI. Thin-section (3–4 mm), axial, coronal, and oblique sagittal plane T1 and T2 fat-saturated imaging is a suitable protocol. Some authors have recommended the use of short tau inversion recovery (STIR) methods for T2 imaging to increase the conspicuity of abnormal signal from adjacent fat; however, STIR has a relatively low signal-to-noise ratio with low tissue contrast and is more susceptible to flow artifacts than other methods. With MRI, normal brachial plexus components demonstrate an intermediate to low signal on all sequences and are surrounded by fat. Abnormal nerves typically appear focally or diffusely enlarged, with deviation or discontinuity. These abnormal nerves demonstrate high T2 signal, and there is associated effacement of perineural fat planes. Postcontrast T1 imaging demonstrates segmental or confluent enhancement in the setting of tumor or infection.
Assessing Metastases
Lung cancer has been reported to be the most common malignancy causing cardiac metastasis. Cardiac and pericardial involvement is found in 17%–31% of patients with bronchogenic carcinoma. Cardiac metastases have been associated with life-threatening complications, including conduction disruptions such as complete atrioventricular block or ventricular fibrillation. Therefore, detection of cardiac metastases can be very important in a patient’s clinical management.
On MRI, cardiac metastases typically demonstrate low T1 ( Fig. 8.7 ) and high T2 signals with gadolinium enhancement. Postcontrast enhancement is necessary to differentiate true tumor from thrombus because chronic thrombus can appear to enhance peripherally. An inversion recovery delayed enhancement sequence with very long inversion time (TI = 600 ms) has been suggested as the best technique to demonstrate true enhancement of tumor, apart from thrombus. Metastatic tumor that has invaded a cardiac chamber will demonstrate a hyperintense filling defect on black blood imaging. In addition, secondary hemodynamic consequences of the mass may be present, including inflow or outflow obstruction of cardiac chambers or valves. Valvular regurgitation in the presence of tumor infiltration could also be identified as flow jets on cine imaging or by using phase contrast techniques.
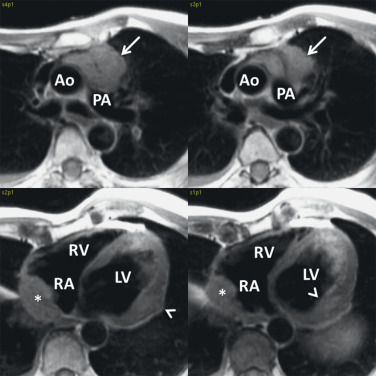
Some studies have suggested that tumor recurrence can be distinguished from radiation fibrosis using T2 imaging. Chronic fibrosis demonstrates a low T2 signal, whereas tumor tissue tends to demonstrate a higher T2 signal. However, a low signal within a lesion does not preclude residual tumor. In addition, a high signal can also be seen with postradiation therapy, early fibrosis, and acute inflammation.
Tumor can be distinguished from atelectasis with postcontrast MRI. Atelectasis usually demonstrates greater signal intensity than tumor due to its better blood supply. Additionally, the peak signal intensity of atelectasis is seen earlier than with tumors. This relationship can change if vascular obstruction is present.
MRI also offers value in characterizing adrenal lesions in patients with lung cancer. Adrenal lesions are detected in up to 21% of lung cancer staging CT examinations, and a high percentage represents benign adenomas. When MRI demonstrates signal loss within the lesion on out-of-phase imaging or fat-saturated sequences, a confident diagnosis of adenoma can be made.
Emerging Roles for Lung MRI in Diffuse Lung Diseases
When conventional MRI sequences are applied to the lungs, there is a rapid decay of signal before it can be observed due to the short T2* of the lungs (~1.5 ms). However, new techniques allow adequate signal acquisition from the lungs by shortening the effective TE. It is possible to image the entire lung parenchyma in vivo during a single breath-hold using a multislice, interleaved, submillisecond TE GRE sequence. The technique involves fractional echo sampling, high bandwidth, and a shortened RF pulse to minimize TE. In addition, using a 3D radial ultrashort TE sequence with TE less than 100 µs enables significantly improved visualization of lung parenchyma. Variations on this 3D radial technique can be used to image lung parenchyma and postcontrast perfusion simultaneously during a single breath-hold.
MRI pulmonary perfusion imaging has correlated to pulmonary function tests in COPD patients. MRI perfusion data, including the pulmonary signal intensity and signal ratio of diseased lung to normal lung, have shown correlation with pulmonary function tests. In addition, significant differences were seen in signal intensity between mild and very severe COPD patients.
The use of hyperpolarized gases, including 3 He and 129 Xe, has improved MRI evaluation of lung ventilation. The use of hyperpolarized 3 He allows for the detection of focal ventilation defects during the approximate 8.16-second breath-hold. Initial studies using hyperpolarized 3 He have shown promise in imaging ventilation defects in patients with exercise-induced bronchospasm. Ventilation defects are also observed in those with asthma and COPD. In asthma, heterogeneous defects have been noted involving the central airways, even in asymptomatic patients.
Unfortunately, hyperpolarized 3 He is expensive, and the supply is limited, given that it is not naturally occurring and is obtained from the decay of tritium. Costs are estimated at $800 to $2000/L of the 3 He gas, depending on the use, which can be prohibitive. The need for a technically complex polarizer and specialized MRI coils and broadband transmit-receive capability has limited the availability of hyperpolarized gas imaging to a few academic centers.
▪
Pulmonary MRA
Sequences Used to Evaluate the Pulmonary Arteries
MRI protocols used for the diagnosis of PE include a combination of noncontrast and contrast-enhanced MRA sequences, DCE perfusion sequences, and steady-state GRE sequences. With respect to maximizing spatial resolution and minimizing acquisition time, 3D contrast-enhanced MRA using parallel imaging and bolus tracking has proven to be the most promising approach and has been reported most extensively in the literature. Using current acceleration methods, it is now possible to carry out whole-chest MRA with 1.0 to 1.5 mm 3 isotropic spatial resolution in approximately 15 to 18 seconds. At our institutions, 0.1 mmol/kg of gadobenate dimeglumine is used, diluted to 30 mL with normal saline, injected at a rate of 1.5 mL/s. This ensures that the contrast bolus remains in the pulmonary arteries throughout the duration of the scan, minimizing the risk of artifact (particularly blurring when using an elliptic, centric, k-space encoding order).
With MRA, it is possible to repeat the acquisition without any concern for ionizing radiation; therefore the 3D MRA sequence is typically repeated two or three times after the injection of IV contrast ( Fig. 8.8 ) to obtain additional steady-state images. These can be acquired using the same parameters as the first-pass acquisition or with parameters adjusted to optimize blood pool to soft tissue contrast. In addition to standard GRE 3D MRA sequences, fat-suppressed T1 GRE acquisitions can be helpful for imaging nonvascular structures. If prescribed breath-holding times are too long for a particular patient, several options are available, including the following: slice thickness can be increased, phase field of view can be decreased, matrix size can be decreased, bandwidth can be increased, and the number of signal averages can be decreased. Cardiac gating is not necessary to achieve high-quality imaging of the pulmonary arteries. The appearance of pulmonary emboli on MRA is similar to CTA, typically appearing as central, low signal intensity filling defects within the center of the pulmonary arteries ( Fig. 8.9 ).