Pulmonary Neoplasms
Jeffrey S. Klein
The Solitary Pulmonary Nodule
The radiologic evaluation of a solitary pulmonary nodule (SPN) remains one of the most common and most difficult diagnostic dilemmas in thoracic radiology (1). The prevalence of SPNs has increased recently as a result of the growing use of MDCT. Before embarking on a detailed diagnostic evaluation of an SPN, one must determine whether a focal opacity seen on the chest radiograph is real or artifactual. When a focal opacity is detected radiographically, efforts should be made to ascertain whether it is truly intrathoracic, which should begin with a careful review of a lateral radiograph to localize the opacity. Densities seen on only a single view may reflect artifacts, skin, chest wall or pleural lesions, or true intrapulmonary nodules. Occasionally, physical examination can reveal a skin lesion that accounts for the opacity. Chest fluoroscopy can be useful to help localize an opacity seen on only a single radiographic projection and can identify the opacity as within the chest wall or alternatively in the lung. If available, dual energy chest radiography with review of the bone image can be used as a problem-solving tool to identify calcified lesions such as healed rib fractures or bone islands, calcified granulomas of lung, or calcified pleural plaques that may produce a nodular opacity on frontal radiographs. Often a limited chest CT focused on the area in question on the chest radiograph is necessary to definitively delineate the location and nature of a focal nodular radiographic opacity.
Comparison chest radiographs, when available, should be reviewed to determine whether nodular opacities were evident previously. An opacity completely stable in size for more than 2 years is considered benign and further evaluation is unnecessary. If there is any concern that a nodule previously seen has enlarged, a chest CT should be obtained for further characterization.
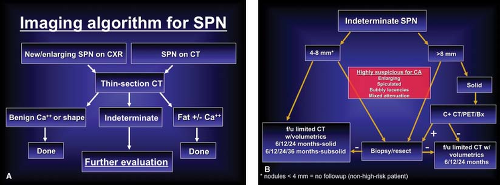
Once a new or enlarging SPN has been identified, the radiologist should initiate a series of investigations to determine whether the nodule has features that are definitely benign, highly suspicious for malignancy, or lacking clear benign or malignant features and therefore indeterminate. This stepwise approach is summarized in Figure 15.1.
Clinical Factors. Before considering the radiologic features used to characterize a lung nodule, several important clinical factors may be helpful in making this distinction. In a patient younger than 35 years, particularly a nonsmoker without a history of malignancy, an SPN is invariably a granuloma, hamartoma, or inflammatory lesion. These nodules can be followed with plain radiographs to confirm their benign nature. Patients older than 35 years, particularly those who are current or recent cigarette smokers, have a significant incidence of malignant SPNs: approximately 50% of radiographically detected noncalcified SPNs in patients older than 50 years are malignant at thoracotomy. Therefore, an SPN in a patient older than 35 years should never be followed radiographically without tissue confirmation unless a benign pattern of calcification or the presence of intralesional fat is identified on radiographs or thin-section CT, or there has been radiographically documented lack of growth over a minimum of 2 years. There are exceptions to this rule: a history of cigarette smoking, prior lung or head-and-neck cancer, or asbestos exposure raises the likelihood for malignancy in a patient with an SPN. Alternatively, if the patient is from an area where histoplasmosis or tuberculosis is endemic, the likelihood of a granuloma is greater; in such patients, a conservative approach may be warranted. Finally, the finding of an SPN in a patient with an extrathoracic malignancy raises the possibility of a solitary pulmonary metastasis. An SPN that arises more than 2 years after the diagnosis of an extrathoracic malignancy is almost always a primary lung tumor rather than a metastasis; breast carcinoma and melanoma are notable exceptions to this rule.
Growth Pattern. Pulmonary malignancies grow at a relatively predictable rate. The growth rate of an SPN is usually expressed as the doubling time, or the time it takes for a nodule to double its volume. For a sphere, this corresponds to a 25% increase in diameter. Although some benign lesions (mostly hamartomas and histoplasmomas) may exhibit a growth rate similar to that of malignant lesions, the absence of growth or an extraordinarily slow or rapid rate of growth of a solid nodule is reliable evidence that an SPN is benign. Studies have shown that bronchogenic carcinoma presenting as a solid SPN has a doubling time of approximately 180 days. Therefore, a doubling time of less than 1 month or greater than 2 years reliably characterizes a solid lesion as benign. Infectious lesions and rapidly growing metastases from choriocarcinoma, seminoma, or osteogenic sarcoma comprise the majority of rapidly growing solitary nodules, whereas lack of growth or a doubling time exceeding 2 years is seen in hamartomas and histoplasmomas. However, there are exceptions to this rule. Giant cell carcinoma, a subtype of large cell carcinoma, and pulmonary carcinosarcomas and blastomas may have a doubling time of less than 1 month. Conversely, malignancies such as some well-differentiated adenocarcinomas or carcinoid tumors may
have a doubling time of greater than 2 years, particularly if they are subsolid (i.e., ground-glass or mixed soft tissue/ground-glass attenuation).
have a doubling time of greater than 2 years, particularly if they are subsolid (i.e., ground-glass or mixed soft tissue/ground-glass attenuation).
In patients with clinical and imaging characteristics suggesting an indeterminate SPN, particularly lesions smaller than 8 mm in diameter, thin-section CT analysis of nodule volume appears to provide a noninvasive method of assessing nodule growth and determining which lesions require biopsy or resection. Published studies have shown that this technique is more accurate than cross-sectional measurements in determining nodule volume and distinguishing between growing malignant SPNs and stable benign lesions (Fig. 15.2). If a decision is made to simply follow an SPN radiologically, either because of a high likelihood of benignity or because the patient cannot tolerate or refuses an invasive diagnostic procedure, the lesion should be followed by limited thin-section CT. The frequency of thin-section CT follow-up of solid lesions 4 to 8 mm in diameter is inversely proportional to the clinical likelihood for malignancy and the lesion diameter. In other words, the larger the lesion and the greater the clinical concern for malignancy, the shorter the follow-up. Recommendations from the Fleischner Society for the follow-up
of incidentally detected small (4 to 8 mm) lung nodules have been published and provide a reasonable guideline for the frequency and length of follow-up (Table 15.1). The only exception to the published recommendations is for subsolid (i.e., ground-glass or mixed solid/ground-glass attenuation) nodules for which a greater than 2-year follow-up is likely necessary given the indolent nature and more typical slow growth of subsolid malignancies.
of incidentally detected small (4 to 8 mm) lung nodules have been published and provide a reasonable guideline for the frequency and length of follow-up (Table 15.1). The only exception to the published recommendations is for subsolid (i.e., ground-glass or mixed solid/ground-glass attenuation) nodules for which a greater than 2-year follow-up is likely necessary given the indolent nature and more typical slow growth of subsolid malignancies.
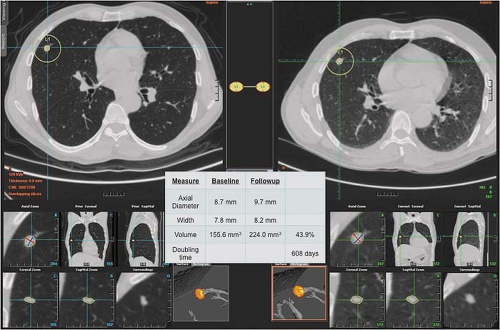 Figure 15.2. Computer-Aided Two- and Three-Dimensional and Volumetric CT Analysis of Solitary Pulmonary Nodule. |
Table 15.1 Fleischner Society Guidelines for the Management of Small (≤8 Mm) Incidental Lung Nodules on Ct | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||
Size. Although size does not reliably discriminate benign from malignant SPNs, the larger the lesion, the greater the likelihood of malignancy. Masses exceeding 4 cm in diameter are usually malignant. However, the converse does not hold true; many pulmonary malignancies are less than 2 cm in diameter at the time of diagnosis, particularly if detected by screening chest CT. In patients with SPNs screened for lung cancer using low-dose CT, nodules <4 mm in diameter have a less than 1% likelihood of malignancy, and therefore most radiologists will not recommend routine follow-up of such lesions unless there is a very high clinical likelihood of malignancy.
Border (Margin, Edge) Characteristics. The appearance of the margin or edge of an SPN is a helpful sign in determining the nature of the lesion. The edge characteristics are best evaluated on thin-section CT, as this technique is considerably more accurate than plain radiographs. A round, smooth nodule is most likely a granuloma or hamartoma, although a rare primary pulmonary malignancy such as a carcinoid tumor, adenocarcinoma, or a solitary metastasis may have a perfectly smooth margin. A notched or lobulated contour may be seen in hamartomas, but malignant lesions including carcinoid tumors and some bronchogenic carcinomas will have a lobulated border. Pathologic examination has shown that the lobulated edge of a malignant nodule represents mounds of tumor extending into the adjacent lung. A spiculated margin is highly suspicious for malignancy (Fig. 15.3). The term corona radiata has been used to describe this appearance, in which linear densities radiate from the edge of a nodule into the adjacent lung. Pathologically, these linear radiations represent reoriented connective tissue (interlobular) septa drawn into the tumor by the cicatrizing (scarring) nature of many malignant lung tumors (Fig. 15.3C). Tumor extension from the nodule, or fibrosis and edema of these connective tissue septa, may thicken these linear densities. However, it has been shown that spiculation is not specific for malignancy, because benign processes that produce cicatrization can have an identical appearance. Benign lesions that may show a spiculated border include lipoid pneumonia, organizing pneumonia, tuberculomas, and the mass lesions of progressive massive fibrosis in complicated silicosis. A peripherally situated pulmonary nodule may contact the costal pleura or interlobar fissure via a linear opacity known as a “pleural tail.” As with the corona radiata, the recognition of this line, while suggestive of malignancy (particularly bronchioloalveolar cell carcinoma), is not specific and may be seen in peripheral granulomas.
There are additional characteristics of the border of an SPN which help identify the nature of the lesion. The presence of small “satellite” nodules around the periphery of a dominant nodule is strongly suggestive of benign disease, particularly granulomatous infection. The identification of feeding and draining vessels emanating from the hilar aspect of an SPN is pathognomonic of a pulmonary arteriovenous malformation (AVM). Contrast-enhanced helical CT scanning through the nodule or MR is diagnostic. A posttraumatic PA pseudoaneurysm will show marked contrast enhancement and contiguity with the feeding artery on CT. The presence of a halo of ground-glass opacity encircling an SPN in an immunocompromised, neutropenic patient should suggest the diagnosis of invasive pulmonary aspergillosis. Finally, a nodule or mass adjacent to an area of pleural thickening, with a “comet tail” of bronchi and vessels entering the hilar aspect of the mass, and associated with lobar volume loss is characteristic of round atelectasis.
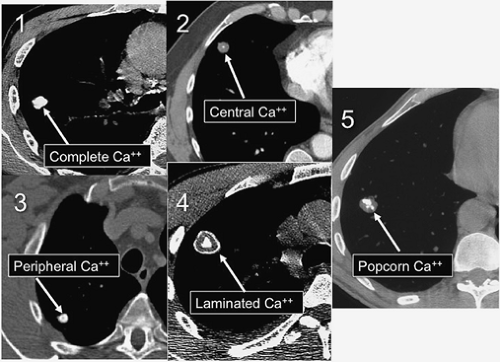
Density. The internal density of an SPN is probably the single most important factor in characterizing the lesion as benign or indeterminate. In general, lesions that are calcified are benign. There are five patterns of calcification that reliably indicate the benignity of an SPN. These patterns can be identified on plain chest radiographs, but thin-section
CT is often necessary to detect and characterize the calcification. Complete or central calcification within an SPN is specific for a healed granuloma from tuberculosis or histoplasmosis. Concentric or laminated calcification indicates a granuloma and allows confident exclusion of neoplasm. Popcorn calcification within a nodule is diagnostic of a pulmonary hamartoma in which the cartilaginous component has calcified.
CT is often necessary to detect and characterize the calcification. Complete or central calcification within an SPN is specific for a healed granuloma from tuberculosis or histoplasmosis. Concentric or laminated calcification indicates a granuloma and allows confident exclusion of neoplasm. Popcorn calcification within a nodule is diagnostic of a pulmonary hamartoma in which the cartilaginous component has calcified.
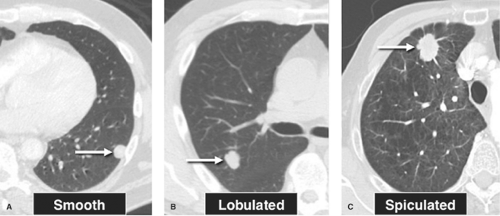 Figure 15.3. Edge or Marginal Characteristics of Solitary Pulmonary Nodules. A. Smooth borders in a granuloma. B. Lobulated contour of a hamartoma. C. Spiculated border in bronchogenic carcinoma. |
It is important to remember that calcification within an SPN is synonymous with a benign lesion only if the calcification follows one of the five patterns of benign calcification shown in Figure 15.4. Approximately 10% of malignant nodules contain calcification on CT. A bronchogenic carcinoma that arises in an area of previous granulomatous infection may engulf a preexisting calcified granuloma as it enlarges. In this situation, the calcification will be eccentric in the nodule, allowing distinction
from a centrally calcified granuloma. Malignant pulmonary neoplasms may demonstrate small or microscopic foci of calcification, particularly adenocarcinomas that produce mucin or psammoma bodies. The rare solitary pulmonary metastasis from osteosarcoma or chondrosarcoma may contain calcium, but the diagnosis in these patients will usually be obvious clinically.
from a centrally calcified granuloma. Malignant pulmonary neoplasms may demonstrate small or microscopic foci of calcification, particularly adenocarcinomas that produce mucin or psammoma bodies. The rare solitary pulmonary metastasis from osteosarcoma or chondrosarcoma may contain calcium, but the diagnosis in these patients will usually be obvious clinically.
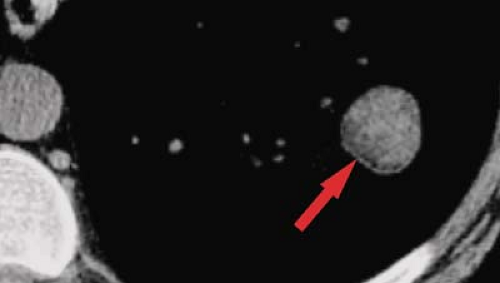 Figure 15.5. Fat in Pulmonary Hamartoma. Cone-down view of unenhanced thin-section CT through left lower lobe nodule shows fat in medial aspect of lesion (arrow), diagnostic of a hamartoma. |
The identification of fat within an SPN is diagnostic of a pulmonary hamartoma (Fig. 15.5). A discussion of the radiographic and CT features of a pulmonary hamartoma can be found in the section “Lesions Presenting as SPNs.”
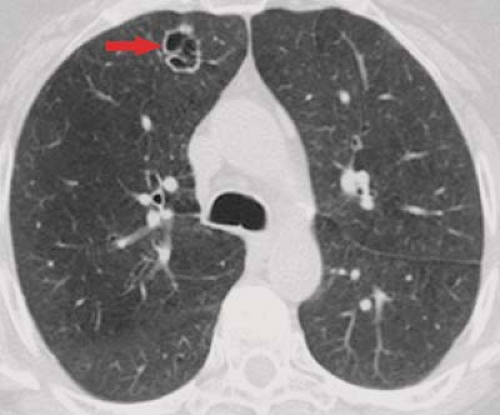
It is important to remember that not all SPNs can be reliably characterized by their internal attenuation characteristics. A lesion with a diameter greater than 3 cm (termed a “mass”), those showing lobulated or spiculated margins, and thick-walled cavitary lesions have a high likelihood of malignancy, regardless of internal density, and almost invariably require tissue diagnosis when detected. Likewise, the demonstration of an air bronchogram or bubbly lucencies within an SPN is highly suspicious for adenocarcinoma (Fig. 15.6), particularly bronchioloalveolar cell subtypes.
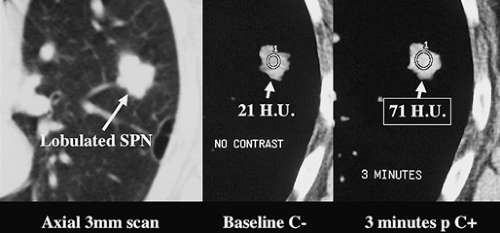
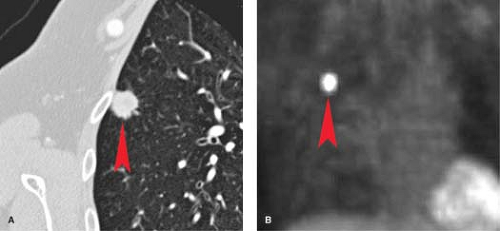
Contrast-Enhanced CT. Several studies have demonstrated the utility of dynamic, contrast-enhanced CT in the evaluation of SPNs, with virtually all malignant lesions demonstrating an increase in attenuation of greater than 15 H after contrast administration (Fig. 15.7) (2). Therefore, lack of significant (>15 H) enhancement of a solid nodule 6 to 30 mm in diameter after IV iodinated contrast effectively excludes malignancy (sensitivity 98%).
PET. PET using fluorine-18-labeled fluorodeoxyglucose (FDG) has shown a high accuracy in the distinction between benign and malignant SPNs (Fig. 15.8) (3). For lesions larger than 10 mm in diameter, the sensitivity and specificity of FDG-PET is 97%, with a specificity of 78%, mostly as a result of inflammatory lesions such as active granulomas that are FDG-avid. False-negative PET studies are seen in patients with lesions smaller than 10 mm in diameter and metabolically hypoactive
lesions such as carcinoid tumor and bronchioloalveolar cell carcinoma. Dual-time point PET, in which images are obtained at both the standard 1 hour and then 2 hours after FDG administration, can improve the sensitivity of PET for nodules with an initial standardized uptake value (SUV) <2.5 (i.e., negative uptake) by showing an increase in SUV on the delayed as compared to the baseline images.
lesions such as carcinoid tumor and bronchioloalveolar cell carcinoma. Dual-time point PET, in which images are obtained at both the standard 1 hour and then 2 hours after FDG administration, can improve the sensitivity of PET for nodules with an initial standardized uptake value (SUV) <2.5 (i.e., negative uptake) by showing an increase in SUV on the delayed as compared to the baseline images.
Management Decisions (Fig. 15.1). Patients with indeterminate SPNs should either have PET, radiologic follow-up or undergo transthoracic biopsy or resection. When the lesion is very likely to be malignant, it is reasonable to forgo biopsy and proceed directly to thoracotomy and resection. However, there are several reasons to perform a preoperative biopsy on an indeterminate SPN. The primary reason to biopsy an indeterminate SPN is to make the diagnosis of a benign lesion, thereby avoiding an unnecessary thoracoscopy or thoracotomy. This would most benefit the patient with a reasonable likelihood of having a benign lesion. Factors suggesting benignity include: age under 35, nonsmoker, patient from an area endemic for tuberculosis or histoplasmosis, nodule smaller than 2 cm with smooth margins, recent symptoms of a lower respiratory infection, and a doubling time of less than 30 days or greater than 2 years. The other major indication for the biopsy of an indeterminate but suspicious SPN is a patient with limited pulmonary reserve who is a poor surgical candidate for pulmonary resection. In these patients, a biopsy can provide a diagnosis and guide nonoperative therapy. Because most SPNs are peripherally situated in the lung, transthoracic needle biopsy (TNB) is the procedure of choice for tissue sampling. Peripheral lesions requiring biopsy that are too small for successful transthoracic needle biopsy (i.e., lesions <5 mm in diameter) can be sampled with video-assisted thoracoscopic surgery (VATS). Patients with SPNs that are centrally situated, with a large bronchus entering the lesion, should undergo transbronchoscopic biopsy.
An SPN that is judged to be benign on the basis of patient age, growth rate, presence of benign calcification, or those with a specific benign diagnosis provided by TNB should be followed with radiographs or CT for a minimum of 2 and preferably 3 years to confirm their benign nature. The radiographic follow-up consists of posteroanterior and lateral chest radiographs if the lesions are radiographically apparent, or limited thin-section CT at 6-month intervals.
Lesions Presenting as SPNs
The differential diagnosis of an SPN is shown in Table 15.2. In addition to bronchogenic carcinoma (particularly adenocarcinoma) and granulomas (e.g., tuberculosis and histoplasmosis), there are a number of entities that may produce an SPN. Many of these entities are discussed elsewhere in the text.
Carcinoid Tumors. While carcinoid tumors may present as SPNs, the majority (80%) are central endobronchial lesions that present with wheezing, atelectasis, or obstructive pneumonitis. A detailed discussion of carcinoid tumors can be found in the section on malignant pulmonary neoplasms.
Pulmonary hamartoma is a misnomer and actually reflects a benign neoplasm composed of an abnormal arrangement of the mesenchymal and epithelial elements found in normal lung. Histologically, these lesions contain cartilage surrounded by fibrous connective tissue, with variable amounts of fat, smooth muscle, and seromucous glands; calcification and ossification are seen in 30%. These tumors are seen most commonly in the fourth and fifth decades of life. Approximately 90% of hamartomas arise within the pulmonary parenchyma, accounting for approximately 5% of all SPNs.
These lesions usually present as incidental findings on chest radiographs. While the diagnosis is often suggested on plain radiographs, CT is obtained in most patients. A confident diagnosis of hamartoma can be made when HRCT shows a nodule smaller than 2.5 cm in diameter demonstrating a smooth or lobulated border and containing focal fat (Fig. 15.5). Calcification, when present, is in the form of multiple clumps of calcium dispersed throughout the lesion (“popcorn” calcification) (Fig. 15.4). As a rule, hamartomas that contain calcium also contain fat. While hamartomas tend to grow slowly, the presence of characteristic thin-section CT findings allows for observation alone. Rapid growth, pulmonary symptoms, or a size larger than 2.5 cm warrants transthoracic biopsy or resection.
Non-Hodgkin Lymphoma. Primary pulmonary lymphomas arising from the bronchus-associated lymphoid tissue (BALT) are low-grade B-cell lymphomas that present in adults
in their fifties. The most common radiographic finding is an SPN or focal airspace opacity. The diagnosis is made by immunohistochemistry and flow cytometry of resected specimens or of aspirated cells obtained by TNB.
in their fifties. The most common radiographic finding is an SPN or focal airspace opacity. The diagnosis is made by immunohistochemistry and flow cytometry of resected specimens or of aspirated cells obtained by TNB.
Table 15.2 Solitary Pulmonary Nodule or Mass | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Granular cell tumor (granular cell myoblastoma) is a benign neoplasm arising from neural elements in the central airways or parenchyma. The skin is the most common site for these tumors. These tumors may present as SPNs but are more commonly seen as endobronchial masses; half of lung lesions present with obstructive pneumonitis because of their endobronchial location (see “Tracheal and Bronchial Masses” section).
Sclerosing Hemangioma. This is a benign epithelial neoplasm that typically affects females and presents as a solitary, smoothly marginated juxtapleural nodule that enhances densely because of its vascular nature. The lesion may contain foci of low attenuation and may be calcified on thin-section CT analysis.
Leiomyoma/Leiomyosarcoma, Fibroma, Neurofibroma. Arising from the smooth muscle of the airways or pulmonary vessels, leiomyomas and leiomyosarcomas are rare neoplasms that present as endobronchial or intrapulmonary lesions with equal frequency. Radiographically, the parenchymal lesions are sharply marginated, smooth or lobulated nodules or masses. The histologic distinction of benign from malignant lesions is difficult. Similarly, fibromas and neurofibromas appearing as SPNs lack distinguishing radiographic features.
Lipomas are rare intrapulmonary lesions that arise more commonly within the tracheobronchial tree to produce atelectasis. The demonstration of fat attenuation on CT is diagnostic.
Hemangiopericytoma is a connective tissue tumor that arises within the lung from the pericyte, a cell associated with the arteriolar and capillary endothelium. On chest radiographs, these lesions are seen as SPNs and are indistinguishable from bronchogenic carcinoma.
Inflammatory myofibroblastic tumor (plasma cell granuloma, inflammatory pseudotumor) of lung refers to a localized chronic inflammatory response to an unknown agent in the lung. It is characterized histologically by an abundance of plasma cells. There are no distinguishing radiographic features.
Lipoid Pneumonia. The inadvertent aspiration of mineral oils ingested by elderly patients to treat constipation may produce a localized pulmonary lesion. Patients with gastroesophageal reflux or disordered swallowing mechanisms are at particular risk. Radiographically, a focal area of airspace opacification or a solid mass may be seen in the lower lobes. A spiculated appearance to the edge of the mass is not uncommon, as the oil may produce a chronic inflammatory reaction in the surrounding lung that leads to fibrosis. While CT can demonstrate fat within the lesion, most patients with the mass-like form of this entity require resection for definitive diagnosis (see Fig. 19.40).
Bronchogenic Cyst. Fluid-filled cystic lesions of the lung may produce an SPN. Intrapulmonary bronchogenic cysts are uncommon causes of SPNs; 90% of these lesions are found in the middle mediastinum. The characteristic finding is a sharply marginated cyst on CT or MR in a young patient, although distinction from an infected bulla, solitary echinococcal cyst, mucocele, or thin-walled lung abscess may be impossible. Superinfection of a lung bulla may produce an SPN or mass. In such patients, the radiographic or CT appearance of an intraparenchymal air-fluid level within a thin-walled localized air collection (usually in an upper lobe), with typical bullous changes in other portions of lung, usually allows for the proper diagnosis.
Focal Organizing Pneumonia. Occasionally, patients who have a resolving pneumonia or even those with a focal mass-like form of cryptogenic organizing pneumonia will have an SPN detected on radiographs or CT. These lesions often show irregular margins and may be PET-positive, thereby showing a significant overlap of findings with those of bronchogenic carcinoma. Sometimes a history of recent lower respiratory tract infection will be present. Radiologic follow-up, perhaps after empiric antibiotic therapy, will allow distinction from malignancy in most patients, although a minority will require surgical resection for definitive diagnosis.
Hematoma/Traumatic Lung Cyst. Blunt or penetrating chest trauma can result in the formation of traumatic lung cysts or hematomas, seen as round opacities often containing air or an air-fluid level.
Bronchogenic Carcinoma
Bronchogenic carcinoma is one of several neoplasms that may arise within the lung (Table 15.3). It is now the leading cause of death from malignancy in the United States and most
industrialized countries for both men and women, having surpassed breast cancer in women in recent years. Although survival rates for lung cancer are poor, radiology plays a central role in diagnosis and management. This section will review the key pathologic, epidemiologic, and radiologic features of bronchogenic carcinoma with an emphasis on the radiologic staging of this disease.
industrialized countries for both men and women, having surpassed breast cancer in women in recent years. Although survival rates for lung cancer are poor, radiology plays a central role in diagnosis and management. This section will review the key pathologic, epidemiologic, and radiologic features of bronchogenic carcinoma with an emphasis on the radiologic staging of this disease.
Table 15.3 Pulmonary Neoplasms | |
|---|---|
|
Cytologic and Pathologic Features
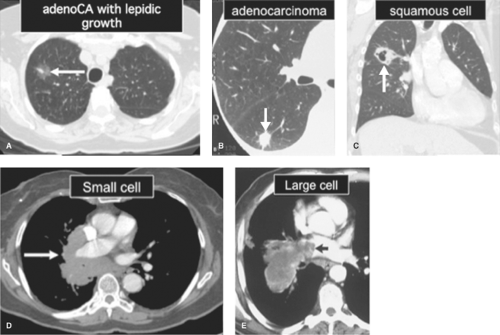
Bronchogenic carcinoma is a malignant neoplasm that arises from the bronchial or alveolar epithelium. Ninety-nine percent of malignant epithelial neoplasms of the lung arise from the bronchi or lung, whereas fewer than 0.5% arise from the trachea. Bronchogenic carcinoma is divided into four main histologic subtypes on the basis of their gross and microscopic features: adenocarcinoma, squamous cell carcinoma, small cell carcinoma, and large cell carcinoma (Table 15.4) (Fig. 15.9).
Adenocarcinoma is the most common type of lung cancer, accounting for approximately one-third of all bronchogenic carcinomas. It is the most common subtype of lung cancer in nonsmokers. Whereas these tumors were once found to occur overwhelmingly in the lung periphery, they are now found in the central portions of the lungs in about one-fourth of cases. These tumors arise from the bronchiolar or alveolar epithelium and have an irregular or spiculated appearance where they invade adjacent lung. Fibrosis in and about the tumor is common. These gross features usually produce an ill-defined pulmonary nodule or mass on chest radiographs (Figs. 15.3C, 15.8B). Histologically, adenocarcinoma demonstrates gland formation and mucin production. A subtype of adenocarcinoma, newly termed adenocarcinoma-in-situ (AIS), previously called bronchioloalveolar cell carcinoma (BAC), has unique pathologic features. This tumor is characterized by growth along preexisting bronchiolar and alveolar walls (“lepidic growth”) without invasion or distortion of alveolar walls, blood vessels, or lymphatics. When localized, AIS appears as a SPN or as a focal area of ground-glass opacity on CT scans (Fig. 15.9A). Diffuse disease, which represents transbronchial (i.e., aerogenous) spread of tumor, may present as airspace opacification simulating pneumonia or as diffuse bilateral nodular airspace opacities.
Squamous cell carcinoma is the second most common subtype of bronchogenic carcinoma, accounting for approximately one-fourth of all cases. This tumor arises centrally within a lobar or segmental bronchus. Grossly, these tumors are polypoid masses that grow into the bronchial lumen while simultaneously invading the bronchial wall. The central location and endobronchial component of the tumor account for the presenting symptoms of cough and hemoptysis and for the common radiographic findings of a hilar mass with or without obstructive pneumonitis or atelectasis. Central necrosis is common in large tumors; cavitation may be seen if communication has occurred between the central portion of the mass and the bronchial lumen (Fig. 15.9C). Histologically, squamous cell carcinoma is characterized by invasion of the bronchial wall by nests of malignant cells with abundant cytoplasm. The formation of keratin pearls and intercellular bridges, seen in well-differentiated tumors, is specific for this tumor.
Small cell carcinoma accounts for 25% of bronchogenic carcinomas and arises centrally within main or lobar bronchi. These tumors are the most malignant neoplasms arising from bronchial neuroendocrine (Kulchitsky) cells and are alternatively referred to as Kulchitsky cell cancers or KCC-3. Typical carcinoid tumors (KCC-1) represent the least malignant type, and atypical carcinoid tumors (KCC-2) are intermediate
in aggressiveness. Small cell carcinomas exhibit a small endobronchial component, invading the bronchial wall and peribronchial tissues early in the course of disease. This produces a hilar or mediastinal mass with extrinsic bronchial compression and obstruction. Invasion of the submucosal and peribronchial lymphatics leads to local lymph node enlargement (Fig. 15.9D) and hematogenous dissemination, which are almost invariable at the time of presentation. Microscopically, these malignant cells are tightly clustered, with nuclei molded together because of the scant amount of cytoplasm. This lesion is distinguished from carcinoid tumor histologically by the presence of mitoses. Electron microscopy demonstrates the presence of intracytoplasmic neurosecretory granules.
in aggressiveness. Small cell carcinomas exhibit a small endobronchial component, invading the bronchial wall and peribronchial tissues early in the course of disease. This produces a hilar or mediastinal mass with extrinsic bronchial compression and obstruction. Invasion of the submucosal and peribronchial lymphatics leads to local lymph node enlargement (Fig. 15.9D) and hematogenous dissemination, which are almost invariable at the time of presentation. Microscopically, these malignant cells are tightly clustered, with nuclei molded together because of the scant amount of cytoplasm. This lesion is distinguished from carcinoid tumor histologically by the presence of mitoses. Electron microscopy demonstrates the presence of intracytoplasmic neurosecretory granules.
Table 15.4 Subtypes of Bronchogenic Carcinoma | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||
Large cell carcinoma accounts for 15% of bronchogenic carcinomas and is occasionally diagnosed when a non-small cell bronchogenic carcinoma lacks the histologic characteristics of squamous cell carcinoma or adenocarcinoma. Histologic features include large cells with abundant cytoplasm and prominent nucleoli. This tumor tends to arise peripherally as a solitary mass and is often large at the time of presentation (Fig. 15.9E).
Epidemiology. The majority of patients with bronchogenic carcinoma are cigarette smokers who are over 40 years of age. Men are most commonly affected, although the percentage of female lung cancer patients has risen steadily in parallel with the increased prevalence of heavy cigarette smoking among women. The overall 5-year survival rate for all patients with lung cancer is 10% to 15%.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree