Overview
Etiology, Prevalence, and Epidemiology
Tuberculosis (TB) is a chronic recurrent contagious infection caused by Mycobacterium tuberculosis. M. tuberculosis is an aerobic, nonmotile, non–spore-forming rod that is highly resistant to drying, acid, and alcohol. It is transmitted person to person via droplet nuclei containing the organism and spread mainly by coughing. The contagiousness of a patient with TB increases with greater extent of disease, the presence of cavitation, the frequency of coughing, and the virulence of the organism. The risk of developing active TB is the greatest in patients with altered host cellular immunity. These include extremes of age, malnutrition, cancer, immunosuppressive therapy (including steroids and anti–tumor necrosis factor drugs), human immunodeficiency virus (HIV) infection, end-stage renal disease, and diabetes.
In 2014 9.6 million people were estimated to develop TB, and 1.5 million died of the disease, 0.4 million of whom were HIV positive. Of the estimated 9.6 million new cases in 2014, 58% were in Southeast Asia and Western Pacific regions and 28% in the Africa region. During 2014 a total of 9407 confirmed TB cases (3.1 cases per 100,000 population) were reported in the United States. Slightly more than half (54%) of US cases were in foreign-born persons. It is estimated that 43 million lives were saved between 2000 and 2014 through effective diagnosis and treatment. To keep the efforts for global elimination of TB, from 2016 the World Health Organization hopes to end the global TB epidemic by implementing the End TB Strategy, which serves to reduce the number of TB deaths by 90% by 2030, cut new cases by 80%, and ensure that no family is burdened with catastrophic costs resulting from TB.
HIV infection is the strongest known risk factor for progression from latent to active TB. Globally, 12% of the 9.6 million new TB cases in 2014 were HIV positive, and TB killed 0.4 million HIV-positive people. The majority of these patients live in countries with limited health care resources—Africa and Asia. The incidence of TB in these countries is increasing. Immune restoration induced by highly active antiretroviral therapy (HAART) in developed countries has considerably improved the outcome of HIV-positive patients and reduced the prevalence of opportunistic infection and TB in these patients. However, HIV-associated TB continues to occur in countries where HAART is widely used and is seen in patients on antiretroviral treatment. Furthermore, HAART may result in paradoxical worsening of TB manifestations in patients with immune reconstitution inflammatory syndrome (IRIS) (see Chapter 15 ).
Clinical Presentation
Patients with active pulmonary TB may be asymptomatic, have mild or progressive dry cough, or present with multiple symptoms, including fever, fatigue, weight loss, night sweats, and productive cough. Elderly patients with pulmonary TB may have different clinical presentations, including more frequent dyspnea and comorbid medical conditions, such as cardiovascular disease, diabetes, and chronic obstructive pulmonary disease.
Pathologic Findings and Pathophysiology
Inhaled mycobacteria are phagocytized by alveolar macrophages, where the organisms multiply and eventually kill the cells. Interaction of macrophages with T lymphocytes results in differentiation of macrophages into epithelioid histiocytes. The epithelioid histiocytes aggregate into small clusters resulting in granulomas. After several weeks granulomas are well formed, and their central portions undergo necrosis. As the disease progresses, individual necrotic foci tend to enlarge and coalesce. The rapid bacillary growth phase is arrested with the development of cell-mediated immunity and delayed-type hypersensitivity at 2 to 10 weeks after the initial infection. The initial focus of parenchymal disease is termed the Ghon focus . The Ghon focus may be microscopic or large enough to be visible radiologically. It either enlarges as disease progresses or, much more commonly, undergoes healing. Healing may result in a visible scar that may be dense and contain foci of calcification. However, commonly there is residual central necrotic material. Although the disease at this stage is inactive, the encapsulated necrotic areas contain viable organisms and are a potential focus for reactivation in later life. Latent TB (LTB) or latent TB infection (LTBI) means that, even though a patient is infected with M. tuberculosis , that the patient does not have active TB (imaging study is normal or seen as scar with dense or calcific lesion); LTBI is not contagious.
During the early stage of infection, organisms commonly spread via lymphatic channels to regional hilar and mediastinal lymph nodes and via the bloodstream to more distant sites in the body. The combination of the Ghon focus and affected lymph nodes is known as the Ranke complex. The course of the disease in lymph nodes is similar to that in the parenchyma, consisting initially of granulomatous inflammation and necrosis followed by fibrosis and calcification. However, the inflammatory reaction is usually much greater in lymph nodes, resulting in lymph node enlargement. Hematogenous dissemination in primary TB is probably common but seldom results in miliary disease.
The initial infection is usually clinically silent. Development of specific immunity is usually adequate to limit further multiplication of the bacilli. Some of the bacilli remain dormant and viable for many years. This condition, known as LTBI, may be detectable only by means of a positive purified protein derivative tuberculin skin test or immunoglobulin-γ release assay, or by the presence of radiologically identifiable calcification at the site of the primary lung infection or in regional lymph nodes. In about 5% of infected individuals immunity is inadequate, and clinically active disease develops within 1 year of infection, a condition known as progressive primary TB. Risk factors for progressive primary disease include immunosuppression (especially HIV infection), extremes of age, or a large inoculation of mycobacteria. In most infected individuals, however, TB remains clinically and microbiologically latent for many years.
In approximately 5% of the infected population (eventually 10%, 5% plus 0.1% per year thereafter), endogenous reactivation of latent infection or reinfection by new strains develops many years after the initial infection. Such postprimary TB frequently is associated with malnutrition, debilitation, or immunosuppression. Pulmonary TB in immunocompetent hosts tends to involve predominantly the apical and posterior segments of the upper lobes and the superior segments of the lower lobes. This localization is likely due to a combination of relatively higher oxygen tension and impaired lymphatic drainage in these regions. As distinct from primary TB, in which healing is the rule, postprimary TB tends to progress. The main abnormalities are progressive extension of inflammation and necrosis, frequently with development of communication with the airways and cavity formation. Endobronchial spread of necrotic material from a cavity may result in tuberculous infection in the same or in other lobes. Hematogenous dissemination may result in miliary TB.
New Concept of Manifestations of the Disease
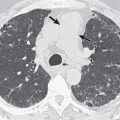
Patients who develop disease after initial exposure are considered to have primary TB. Patients who develop disease as a result of reactivation of a previous focus of TB or reinfection by new strains are considered to have postprimary TB. Traditionally, it was believed that the clinical, pathologic, and radiologic manifestations of postprimary TB were quite distinct from those of primary TB. However, more recent studies based on DNA fingerprinting suggest that the radiographic features are often similar in patients who apparently have primary disease and those who have postprimary TB. In addition, a recent study revealed that the most common radiographic findings in primary pulmonary TB by recent infection in previously healthy adolescents were consolidation, nodules, and cavities in the upper lung, which were thought to be radiographic findings of reactivation pulmonary TB by remote infection ( Fig. 10.1 ). Time from acquisition of infection to development of clinical disease does not reliably predict the radiographic appearance of TB. The only independent predictor of radiographic appearance is integrity of the host immune response. Patients with normal response tend to show parenchymal granulomatous inflammation with slowly progressive nodularity and cavitation, whereas patients with immunodeficiency tend to develop lymphadenopathy, lower lobe parenchymal consolidation, miliary dissemination, and pleural effusion. Therefore the traditional concept of differences in imaging findings between primary and postprimary TB should be changed, and new radiologic classifications by lesion sites—namely, parenchymal, lymph node, airway, and pleural TB—are proposed in this textbook.
- •
Conditions associated with increased risk of tuberculosis
- •
Old age (>70 years), malnutrition, cancer, immunosuppressive therapy, HIV infection, end-stage renal disease, and diabetes
- •
- •
Characteristic manifestations
- •
Granulomatous inflammation—that is, necrotic focus with surrounding epithelioid histiocytes and multinucleated giant cells
- •
- •
Primary infection
- •
Initial parenchymal lesion (Ghon focus) and lymph node inflammation (Ranke complex), usually heal but remain as a dormant infection (latent tuberculosis infection)
- •
- •
Postprimary tuberculosis
- •
Eventually, 10% of patients with primary infection develop reactivation or reinfection by new strains.
- •
- •
New concept
- •
The only independent predictor of radiographic appearance of tuberculous lung infection is integrity of the host immune response, not time from acquisition of infection to development of clinical disease.
- •

Radiographic Findings
The typical radiographic manifestation of parenchymal TB in immunocompetent hosts consists of focal or patchy heterogeneous consolidation involving the apical and posterior segments of the upper lobes and the superior segments of the lower lobes ( Fig. 10.2 ; see Fig. 10.1 ). Another common finding is the presence of poorly defined nodules and linear opacities (fibronodular pattern of TB). In one review of the radiographic features of 158 patients with postprimary TB, approximately 55% presented with consolidation, 25% with a fibronodular pattern, and 5% with a mixed pattern. Single or multiple cavities are evident radiographically in 20% to 45% of patients ( Figs. 10.3 and 10.4 ). Air-fluid levels are seen in 10% to 20% of tuberculous cavities. In approximately 85% of patients the cavities involve the apical or posterior segments of the upper lobes and, in approximately 10%, the superior segments of the lower lobes. Endobronchial spread, manifested as 4- to 10-mm-diameter nodules distant from the site of the cavity, is evident radiographically in 10% to 20% of cases (see Fig. 10.3 ).



In approximately 5% of patients with postprimary TB, the main manifestation is a tuberculoma, defined as a sharply marginated round or oval lesion measuring 0.5 to 4.0 cm in diameter ( Fig. 10.5 ). Histologically, the central part of the tuberculoma consists of caseous material and the periphery of epithelioid histiocytes and multinucleated giant cells and a variable amount of collagen. Tuberculomas usually occur in the upper lobes; approximately 80% are single and 20% are multiple. Satellite nodules histologically identical to the larger focus of disease and measuring 1 to 5 mm in diameter are present in most cases (see Fig. 10.5 ).

In immunocompromised hosts, pulmonary TB manifests as miliary TB, hilar or mediastinal lymphadenopathy, and pleural effusion. Hilar or mediastinal lymphadenopathy is uncommon in postprimary TB, being seen in approximately 5% to 10% of patients. Pleural effusion, typically unilateral, occurs in 15% to 20% of patients. Although pleural effusion is usually associated with parenchymal abnormalities ( Fig. 10.6 ), it may be the only radiologic manifestation of TB ( Fig. 10.7 ). Pleural effusion can be caused by rupture of a tuberculous cavity into the pleural space. This may result in the formation of tuberculous empyema and, occasionally, a bronchopleural fistula with pleural air-fluid level.


Miliary spread of TB can occur in both primary and recurrent disease ( Figs. 10.8 and 10.9 ). In the latter situation it may be seen in association with typical parenchymal changes as described previously or may be the sole pulmonary abnormality. Each focus of miliary infection results in local granulomas, which when well developed consist of a region of central necrosis surrounded by a relatively well-delimited rim of epithelioid histiocytes and fibrous tissue (see Fig. 10.9 ).


Pulmonary TB may result in a number of complications and sequelae. Parenchymal and airway complications include acute respiratory distress syndrome (ARDS), extensive lung destruction and cicatrization, multiple cystic lung lesions ( Fig. 10.10 ), aspergilloma, bronchiectasis, tracheobronchial stenosis ( Fig. 10.11 ), and broncholithiasis. The radiologic manifestations of ARDS secondary to TB include extensive bilateral ground-glass opacities or consolidation superimposed on findings of miliary or endobronchial spread of TB. Multiple cystic lesions may develop in patients recovering from ARDS or in patients with extensive consolidation resulting from TB. The cystic lesions may resemble pneumatoceles or bullae. They may resolve over several months or persist (see Fig. 10.10 ).


Vascular complications of pulmonary TB include pulmonary and bronchial arteritis and thrombosis, bronchial artery pseudoaneurysm, and Rasmussen aneurysm ( Fig. 10.12 ). Rasmussen aneurysm is a pseudoaneurysm that results from weakening of the pulmonary artery wall by adjacent cavitary TB. Mediastinal complications include esophagomediastinal or esophagobronchial fistula, constrictive pericarditis ( Fig. 10.13 ), and fibrosing mediastinitis. Pleural complications include tuberculous pleurisy and empyema, empyema necessitatis, fibrothorax, pneumothorax, and bronchopleural fistula. Empyema necessitatis ( Fig. 10.14 ) results from leakage of tuberculous empyema through the parietal pleura with discharge of its contents into the subcutaneous tissues of the chest wall or, less commonly, pericardium, vertebral column, or esophagus. The main chest wall complications are tuberculous osteomyelitis and chondritis, tuberculous spondylitis ( Fig. 10.15 ), and empyema necessitatis.
- •
Immunocompromised hosts
- •
Lymph node enlargement, pleural effusion, miliary dissemination, lower lobe consolidation
- •
- •
Immunocompetent hosts
- •
Focal or patchy consolidation in apical and posterior segments of upper lobes and superior segments of lower lobes
- •
Cavitation (20%–45%)
- •
Small nodules away from primary lesion (20%–25%)
- •
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree