The term quality assurance (QA) describes a program that is designed to control and maintain the standard of quality set for that program. For radiation oncology, a quality assurance program is essentially a set of policies and procedures to maintain the quality of patient care. The general criteria or standards of quality are usually set collectively by the profession. It is expected that a QA program designed specifically for an institution will meet those standards.
Model QA programs in radiation oncology have been proposed by professional organizations such as the American College of Radiology (ACR), the American Society for Radiation Oncology (ASTRO), and the American Association of Physicists in Medicine (AAPM). These programs incorporate many of the standards and criteria developed by the National Council on Radiation Protection and Measurements (NCRP), the International Commission on Radiation Units and Measurements (ICRU), the International Commission on Radiological Protection (ICRP), and the International Electrotechnical Commission (IEC). In addition, mandatory programs with QA components have been instituted by the Nuclear Regulatory Commission (NRC) and the individual states. The Joint Commission on Accreditation of Healthcare Organization (JCAHO) has also set minimum standards of QA that are required of hospitals seeking accreditation.
Despite the many standard-setting bodies and the regulatory agencies, the standards of radiation oncology practice are quite varied across the United States. A classic patterns of care study in 1985 (
1), using Hodgkin disease, prostate cancer, and cervix cancer as examples, showed correlations between patient outcome and facility equipment, structure, technical support, and physician characteristics. These data underscore the importance of quality assurance in providing patients the best chance for cure.
The major reason for the lack of commitment to QA by many institutions is financial. An adequate QA program requires increased staffing and up-to-date equipment, both of which can be expensive. According to the analysis by Peters (
2), the total cost of the QA program in radiation therapy amounts to approximately 3% of the annual billing for combined technical and professional charges. Because QA programs are voluntary (with the exception of the NRC or the state-mandated component), the only incentive to establishing these programs is a desire to practice good radiation therapy or avoid malpractice suits. However, the latter has not been a sufficient deterrent to effect change.
17.1. GOALS
The Inter-Society Council for Radiation Oncology specifies the goals of a QA program in its 1991
Blue Book (
3):
The purpose of a Quality Assurance Program is the objective, systematic monitoring of the quality and appropriateness of patient care. Such a program is essential for all activities in Radiation Oncology. The Quality Assurance Program should be related to structure, process and outcome, all of which can be measured. Structure includes the staff, equipment and facility. Process covers the pre- and post-treatment evaluations and the actual treatment application. Outcome is documented by the frequency of accomplishing stated objectives, usually tumor control, and by the frequency and seriousness of treatment-induced sequelae.
That “such a program is essential for all activities in radiation oncology” emphasizes the need for a comprehensive QA program that includes administrative, clinical, physical, and technical aspects of radiation oncology. Operationally, no single person has the expertise to cover all these areas. Therefore, teamwork is essential among administrators, radiation oncologists, nurses,
medical physicists, and therapy technologists. For a QA program to be effective, all the staff involved with the radiation oncology service must be well coordinated and committed to QA.
The 1991 Blue Book has been updated by ASTRO in collaboration with other major societies representing physicians, medical physicists, medical dosimetrists, radiation therapists, nurses, and radiation oncology administrators (
4). This new book, entitled
Safety Is No Accident: A Framework for Quality Radiation Oncology and Care, was published in 2012 and is available online at: https://www.astro.org/Clinical-Practice/Patient-Safety/Blue-Book/bfp/index.html#/60. The new document provides a blue print for contemporary radiation oncology facilities in terms of structure, process, and personnel requirements.
17.2. PHYSICS STAFFING
The physics component of quality assurance in radiation oncology is one of the major responsibilities of the radiation physicist. Adequate physics staffing, in proportion to patient load and equipment, is required to carry out these responsibilities properly. The
Blue Book (
4) recommends at least one physicist per center. The number of additional physicists should be calculated based on the patient load treated annually, complexity, and sophistication of equipment, and the extent of academic functions such as teaching, research, and administration (
Tables 17.1A and
17.1B).
Many of the clinical physics tasks that have been traditionally performed by physicists can be delegated to dosimetrists or physics assistants. For example, dosimetrists can assist in routine QA checks, computer treatment planning, monitor unit calculations and brachytherapy source preparations. A physicist in this case has a role in establishing the procedures, directing the activities, and reviewing the results.
In the area of treatment planning,
Figure 17.1 illustrates how physics support has traditionally existed in this country. Arrangement A, in which the physician practically works alone or rarely seeks consultation from the physics team, is obviously not appropriate and is contrary to the concept of a multidisciplinary approach to radiation oncology. Arrangement B is not satisfactory either but is still prevalent in some institutions. There may be several reasons why an essential member of the team, the physicist, is excluded in this case from the clinical process. Economics is one reason, as physicists are usually higher salaried than the dosimetrists. Other reasons may include having physicists on staff who lack clinical training or do not have a well-defined role in the clinic. Nonetheless, arrangement C is probably the best approach, as it involves teamwork among personnel whose responsibilities are matched with their credentials.
The new Blue Book recommendation on dosimetrist staffing is approximately 1 per 250 patients treated annually. In some institutions, dosimetrists perform physics work only, whereas in others they also do simulations. The relative proportion of a dosimetrist’s efforts to various tasks is dictated by the individual needs of the department, the scope of the physics activities, and the extent of other physics and technical support available.
A. TRAINING
Besides the adequacy of physics staffing in terms of number of personnel in relation to the patient load or equipment, education and training of these personnel are of critical importance. The greatest weakness in this regard has been the physicist’s training. Many physicists are hired with less than adequate clinical training. Structured clinical training programs such as hospital-based residency training for physicists was nonexistent until the beginning of 1990s. Even now, the available accredited residency programs are not enough to meet the demand. As a result, there is still an unchecked influx of inadequately trained physicists into the field.
More physics residency programs are being instituted, although at a much slower pace than needed. It is hoped that eventually all clinical medical physicists would be required to go through nationally accredited residency programs and be duly trained and board-certified before assuming independent clinical responsibilities. It is encouraging to note that the American Board of Radiology (http://www.theabr.org)—the certifying board for radiological physicists— has decided that “Beginning in 2014 … candidates must be enrolled in or have completed a CAMPEP [Commission on Accreditation of Medical Physics Educational Programs] accredited residency program.”
Just as formalized clinical training is important for physicians and physicists, it is also important for dosimetrists. The American Association of Medical Dosimetrists (AAMD) (http://medicaldosimetry.org/) has formalized the training of dosimetrists by establishing training curricula, accreditation, and professional certification. Additional information regarding dosimetry certification through the Medical Dosimetrists Certification Board (MDCB) may be found at www.mdcb.org.
B. QUALIFICATIONS
Qualifications of a clinical medical physicist have been debated in the past, but a consensus has eventually developed among the various national organizations. The AAPM has defined a Qualified Medical Physicist (QMP) as one who meets each of the following credentials:
Has earned a master’s or doctoral degree in physics, medical physics, biophysics, radiological physics, medical health physics, or equivalent disciplines from an accredited college or university
Has been granted certification in the specific subfield(s) of medical physics with its associated medical health physics aspects by an appropriate national certifying body and abides by the certifying body’s requirements for continuing education (AAPM Professional Policy 1-H, http://www.aapm.org/org/policies/details.asp?id=316&type=PP).
A medical dosimetrist has a minimum of a high school diploma and a certification by the MDCB. A formal definition and expectations of a qualified medical dosimetrist are provided by the AAMD:
A Qualified Medical Dosimetrist is an individual who is competent to practice under the supervision of a qualified physician and qualified medical physicist. This individual uses critical thinking and problem solving skills as well as exercises discretion and judgment in the performance of medical dosimetry procedures.
It is expected that an individual will hold him/herself qualified to practice in medical dosimetry only when the knowledge and skills to perform dosimetric tasks has been established.
An individual shall be considered eligible to practice if he/she is certified by the Medical Dosimetrist Certification Board (MDCB). The MDCB will require a Baccalaureate Degree to sit for their exam by the year 2017 and the AAMD fully supports that educational level for new candidates.
The American Association of Medical Dosimetrists (AAMD) regards that personnel practicing in Medical Dosimetry meet, at a minimum, certification provided by the Medical Dosimetry Certification Board (MDCB). Accordingly, the CMD (Certified Medical Dosimetrist) is recognized as the appropriate credential for the Medical Dosimetrist.
(http://medicaldosimetry.org/generalinformation/definition.cfm)
C. ROLES AND RESPONSIBILITIES
The roles and responsibilities of the physics team have been discussed in the literature (
5,
6) and are summarized in
Table 17.2. As emphasized previously, the physicist must direct the physics team and assume full responsibility of the physics data and procedures applied to the patients, irrespective of whether these activities are carried out by the physicist, dosimetrist, or other personnel. This unambiguous responsibility of physics procedures by the physicist is based on the same rationale as the physician’s control of medical prescription. Nonconformity to this principle can pose a serious risk to the patient.
The radiation oncologist undoubtedly has the overall responsibility for the conduct of the entire treatment process. Because of that role, it is his or her responsibility to ensure that an adequate and competent physics team is in place and that the roles of different personnel on the team are appropriately defined. It should be recognized that inadequacy of physics support translates into substandard or less than optimal patient care.
Calibration of radiation generators or sources is the exclusive responsibility of the medical physicist. No other personnel have the expertise to perform this most critical function. Because of the absolute necessity of the calibration procedure before the machine can be released for patient treatment, all institutions manage to acquire physics support at least sufficient to provide periodic calibration of the radiation equipment. However, these periodic calibrations, outside of a well-structured quality assurance program, are inadequate and cannot ensure continued safety of machine operation on a daily basis (
7).
Next to calibration and quality assurance of radiation equipment is the physicist’s role in treatment planning. Although the treatment-planning process involves sophisticated physics concepts in designing and optimizing patient treatments, most institutions do not involve physicists sufficiently in this process. As discussed previously (
Fig. 17.1), some physicians tend to work with dosimetrists without any significant input from the physicist to design treatment plans. It should be realized that the absence of a physicist from the treatment-planning process takes away an important element of quality control, namely the optimization and scientific authentication of the treatment plan. The physicist’s direct involvement in the treatment-planning process is possible only if the consultation is sought by the radiation oncologist. If the latter is not accustomed, by training or experience, to such interactions, the physicist is not brought into the treatment-planning loop. Consequently, to the detriment of the patient, an important member of the treatment-planning team is bypassed.
Physicist’s consultation in treatment planning is as important as other consultations, such as those sought from the medical oncologist, the surgeon, or the radiologist. One way to prevent bypassing of the physics consultation is to have each patient assigned to a physicist who is available at the time of simulation to assist the radiation oncologist in formulating the best possible treatment plan. Subsequent physics work is the designated physicist’s responsibility, although he or she may be assisted by the dosimetrist or other technical personnel. The final treatment plan is approved by the radiation oncologist after discussing the plan with the physicist. Also, the physicist is present at the time of first treatment and subsequent treatments, if needed, to ensure proper implementation of the plan.
Not all the clinical physics procedures need to be performed by physicists. Many of the technical tasks can be delegated to dosimetrists so that physicists can devote time to essential developmental activities. Every radiation oncology department needs to develop new programs as well as revise the old ones to keep current with advancements in the field. Responsibility often rests with the physicist to implement these advances while maintaining the quality of care. It is, therefore, important to recognize the need for providing sufficient technical support to the physicist for a proper fulfillment of his or her role.
17.4. DOSIMETRIC ACCURACY
Available evidence for effectively treating certain types of cancers points to the need for an accuracy of approximately ±5% in dose delivery (
8,
9). This is indeed a very stringent requirement, considering the uncertainties in equipment calibration, treatment planning, and patient setup. Further reduction in the dose accuracy limit will be not only very difficult, but also probably of marginal value.
Calculation of overall uncertainty in a radiation therapy procedure is a complex problem, because some errors are random while others can be systematic. Loevinger and Loftus (
8) have proposed a model in which the random and systematic errors are statistically treated in the same way. Individual uncertainties are represented by standard deviations that are then added in quadrature to determine the cumulative uncertainty. The combined standard deviation may be multiplied by two to obtain an uncertainty with a 95% confidence interval.
Table 17.3 gives an estimate of uncertainty in the calibration of a treatment beam with a field ion chamber (e.g., 0.6 cm
3 Farmer-type chamber). The analysis shows that an ion chamber suitable for calibrating radiation therapy beams and provided with a
60Co exposure calibration factor from an accredited dosimetry calibration laboratory (ADCL) has a cumulative uncertainty of approximately 1.5% (two standard deviations). Calibration of beams with this chamber will
introduce additional uncertainties such as in the measurement procedure and in the parameters of the dosimetry protocol. The overall uncertainty of the treatment beam calibration using current protocols is estimated to be about 2.5% under optimal conditions (
8).
Table 17.4 gives uncertainties in the various steps involved in delivering a certain dose to a patient at a reference point such as at the isocenter. The estimate of the uncertainties in these steps is approximate and arrived at by considering the various procedures as they may be typically carried out. These uncertainties could be further refined and broadened to include uncertainties in the dose distribution within the target volume and the surrounding structures. A QA program must address not only the issues of random and systematic errors inherent in the procedures, but also the possibilities of human error such as in reading an instrument, selecting a treatment parameter, making arithmetic computations, or interpreting a treatment plan. Although human errors cannot be eliminated altogether, the probability of their occurrence can be minimized by a well-designed QA program. An undue relaxation of a QA program or the lack of it can be construed as professional negligence.
17.5. EQUIPMENT SPECIFICATIONS
Acquisition of a major piece of equipment involves many steps: justification of need, market evaluation of different makes and models, checks of vendors’ business relations and service record, calling up users for their opinions, writing bid specifications, making a final evaluation, and doing price negotiations. Even if the institution does not require closed bids, it is important to prepare detailed specifications and obtain a formal written response from the vendor before deciding on the purchase.
Most vendors list their equipment specifications in company brochures that are available on request. These specifications should be carefully compared with other vendors’ specifications.
Formal bid specifications can then be written for the product that most closely meets the institution’s needs. For an impartial bid process, the specifications should be as generic as possible, so that all major vendors have a fair chance of responding to the bids. Specifications that the institution considers essential must be identified so that vendors who cannot meet those specifications do not have to go through the process of answering the bid. If a particular system is specified to meet a certain function, vendors should have the opportunity to suggest alternative systems with equivalent or better specifications.
The purchase of radiation therapy equipment is usually a shared responsibility between the radiation oncologist, the physicist, and the hospital administrator. The physicist’s role is primarily to write technical specifications, although most participate in the whole decision process.
The specifications are written in a suitable format so that the vendors can respond to them item by item. Because the vendors’ responses are legally binding, clarification should be sought if response to a particular item is not clear. Also, if a specification in a company’s brochure falls short of a desired specification, negotiations may be carried out with the vendor to improve the specification in question. Many improvements in the accelerator technology have occurred as a result of customer demand for better specifications.
Certain specifications in regard to beam characteristics and acceptance criteria require carefully stated definitions and methods of measurement. The specifications should clearly spell out these details, especially when conflicting definitions or criteria exist in the literature. As far as possible, the specifications should follow national or international terminology and guidelines unless a special need exists to justify deviations from the accepted standards.
17.6. ACCEPTANCE TESTING
Unless the vendor has agreed to a written set of specifications, the customer has no recourse but to follow the vendor’s acceptance test procedures. These procedures are set up by the company to demonstrate that the product meets the specifications contained in its brochures and satisfies the legal requirements of equipment safety.
If a set of bid specifications was agreed on before the machine was purchased, then the vendor is obligated to satisfy all the specifications and criteria contained in the purchase contract. In practice, the vendor first performs all the tests in accordance with the company’s procedure manual. Any deviations or additions stipulated in the bid specifications are then addressed to complete the acceptance testing process.
As a general rule, acceptance testing is required on any piece of equipment that is used in conjunction with patient treatments. Whereas formal testing procedures have been developed for major equipment (linear accelerators, simulators, brachytherapy sources, etc.), these have to be improvised for other equipment. The guiding principle is that any equipment to be used for patients must be tested to ensure that it meets its performance specifications and safety standards.
A. LINEAR ACCELERATOR
A linear accelerator is a sophisticated piece of equipment that requires several months for installation, acceptance testing, and commissioning. Whereas installation is carried out by the vendor personnel, the acceptance testing and commissioning are the responsibility of the institution’s physicist. Patient treatments do not begin until the unit has been commissioned; that is, the machine tested to be acceptable and sufficient data have been acquired to permit treatment planning and dose calculations for patient treatments.
A.1. Radiation Survey
As soon as the installation has reached a stage at which a radiation beam can be generated, the physicist is called on to perform a preliminary radiation survey of the treatment facility (
Chapter 16). The survey is evaluated to ensure that during the testing of the machine the exposure levels outside the room will not exceed permissible limits, considering the dose rate output, machine on time, use factors, and occupancy factors for the surrounding areas. A preliminary calibration of the machine output (cGy/MU) is needed to determine the expected dose levels as a function of machine output (MU/min).
After completion of the installation, a formal radiation protection survey is carried out, including the measurement of head leakage; area survey; and tests of interlocks, warning lights, and emergency switches. The survey is evaluated for conditions that are expected to exist in the clinical use of the machine, for example, workload, use factors, and occupancy factors.
A.2. Jaw Symmetry
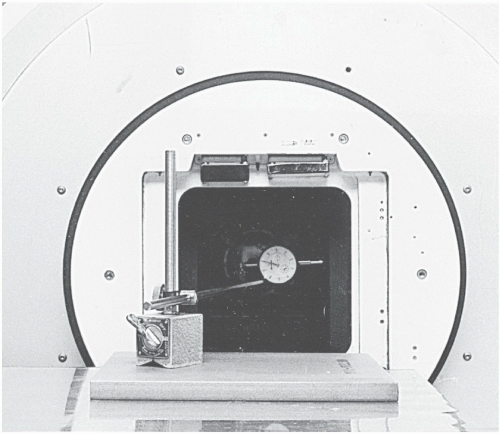
One of the methods of checking jaw symmetry is with a machinist’s dial indicator (
Fig. 17.2). With the gantry pointing horizontally and the jaws open to a large field, the feeler of the dial indicator is made to touch the face of one of the jaws and the indicator’s reading is noted. The collimator is then rotated through 180 degrees and the reading is taken with the feeler now resting on the opposite jaw. A leveling device is used to set the collimator angles for these measurements. The symmetry error is one-half of the difference between the two readings of the dial indicator. The procedure is repeated for the second set of jaws. The symmetry error of the collimator jaws is typically less than 1 mm.
A.3. Coincidence
COLLIMATOR AXIS, LIGHT BEAM AXIS, AND CROSS-HAIRS. With a graph paper taped on the table and the gantry vertical, turn on the field light to obtain a rectangular field. Mark the edges of the light field, intersection of the diagonals, and position of the cross-hair images. Rotate the collimator through 180 degrees and check the coincidence of (a) the light field edges and (b) the intersection of diagonals and the position of cross-hair images. If significant misalignment exists, the field light and cross-hairs should be adjusted to bring about the desired coincidence.
LIGHT BEAM WITH X-RAY BEAM. Place a ready pack film on the table at the source to axis distance (SAD). With the collimator angle set at 0 degrees, obtain a rectangular or square light field and mark the edges with a radiopaque object or a ballpoint pen by drawing lines on the film jacket with sufficient pressure to scratch the emulsion. The film orientation and the collimator angle are noted. A plastic sheet, thick enough to provide maximum electronic buildup, is placed over the film without disturbing its position. This is done to eliminate the perturbing influence of the incident electron contamination. The film is exposed to obtain an optical density in the linear range of its sensitometric curve, usually around 1. Two more exposures at collimator angles of ±90 degrees are made using fresh areas of the same film or on a second film. The film is processed in an automatic rapid processor.
The alignment between the x-ray beam edges (corresponding to an optical density of 50% relative to that on central axis) and the light beam marks can be checked visually or by cross-beam optical density profiles. A typical alignment film is shown in
Figure 17.3. For acceptance testing, the above process should be repeated at 0 degrees, 90 degrees, 180 degrees, and 270 degrees angulation of the gantry.
According to the AAPM guidelines (TG-142) (
10), the alignment between the light beam and the x-ray beam should be within ±2 mm or 1% on a side.
A.4. Mechanical Isocenter
Mechanical isocenter is the intersection point of the axis of rotation of the collimator and the axis of rotation of the gantry. Due to heavy weight, the gantry frame may flex during rotation. This may cause the axis of the gantry rotation to miss the axis of the collimator rotation, thereby creating an uncertainty in the position of the isocenter.
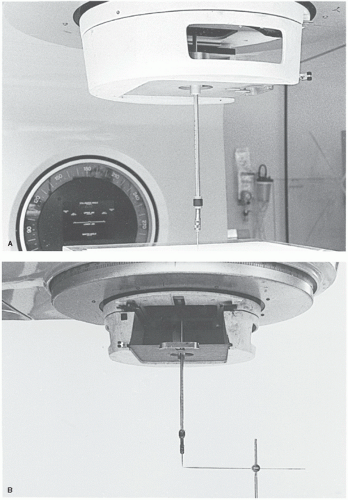
COLLIMATOR ROTATION. Attach a piece of graph paper on a flat surface of a plastic sheet and mark an intersection point of two graph lines (center point). Using the distance-measuring rod attached to the accessory mount, place the center point of the graph at the assumed isocenter (point P). Reverse the distance rod and attach an adjustable pointer device with a sharp point, called the center finder or wiggler, to its distal end (
Fig. 17.4A). Starting with a 0-degree angle, rotate the collimator to +90 degrees and note the maximum displacement of the wiggler tip from point P in the
X and
Y directions. Tap the wiggler point to move it in the
X direction through half the distance from point P and then tap the plastic sheet to bring point P under the wiggler tip. Repeat the procedure for the
Y axis. Rotate the collimator to ±90 degrees and repeat the whole procedure. By iterative adjustment of the wiggler tip and point P, the displacement of the wiggler tip from point P can be minimized as the collimator is rotated. For an acceptable alignment, the isocenter should stay within a 2-mm-diameter circle when the collimator is rotated through its full range of rotation.
GANTRY ROTATION. With the wiggler point positioned at the isocenter as determined previously, another horizontal rod with a fine point is held in a ring stand so that the two points coincide as best as possible (
Fig. 17.4B). The stand for the horizontal rod should rest on the couch near its
end so that there is no possibility of gantry collision with the couch or the apparatus. By moving the gantry through 360 degrees, the displacement between the wiggler point and the horizontal rod point is visually noted and measured. The tolerance of the isocenter motion with full gantry rotation is ±1 mm.
A.5. Radiation Isocenter
COLLIMATOR. With the gantry vertical, place a ready pack film flat on the tabletop at the SAD. Open the upper jaws of the collimator wide and close the lower jaws to obtain a narrow slit of
minimum possible width. Place a buildup sheet on top of the film. By rotating the collimator through a number of different angles, the film is exposed to obtain an optical density of about 1. The interval between angles should be such that six to seven exposures can be made to cover full rotation of the collimator without overlaps. Using a new film, repeat the above process with the lower jaws open and the upper jaws closed to a narrow slit.
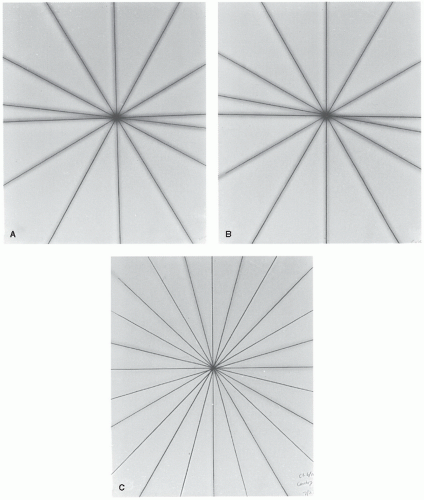
The processed films will show star patterns, with a dark central region (
Fig. 17.5A). By using a ballpoint pen or another film marker with a fine point, lines may be drawn through the middle of the slit images to define more clearly the intersection point(s) of the slit images. For an acceptable result, all the lines should intersect or pass within a 2-mm-diameter circle.
TREATMENT TABLE. Place a film on the tabletop at the SAD. Open the upper collimator jaws wide and close down the lower jaws to a narrow slit. Place a buildup sheet on top of the film. Make six to seven exposures on the same film with the table rotated through a series of different angles to cover the full range of couch rotation. Some exposures may have to be staggered to avoid overlap of images. A star pattern (
Fig. 17.5B) on the processed film should ideally show all the slit images intersecting at one point, the radiation isocenter. Acceptable specification requires that
all the lines should intersect or pass within a 2-mm-diameter circle. Stricter specification may be required for a unit designated for stereotactic radiosurgery.
GANTRY. A ready pack film, sandwiched between two plastic sheets (e.g., acrylic or clear polystyrene), is placed on the table so that the plane of the film is perpendicular to the plane of couch top and contains the beam central axis for all gantry angles. Create a slit of beam parallel to the gantry axis of rotation. Make 12 exposures on the same film with the gantry rotated between exposures. To avoid overlaps, the first six exposures may be made at 30-degree intervals, the next one at 45 degrees beyond, and the subsequent exposures successively 30 degrees apart.
The gantry star pattern (
Fig. 17.5C) should show the lines intersecting or passing within a 2-mm-diameter circle centered around the presumed radiation isocenter.
A.6. Multiple Beam Alignment Check
When a patient is treated with more than one beam, misalignment between beams can occur due to any of the causes discussed previously. Lutz et al. (
11) have recommended a test procedure that can detect simultaneously three general causes of beam misalignment: (a) focal spot displacement, (b) asymmetry of collimator jaws, and (c) displacement in the collimator rotation axis or the gantry rotation axis. This method is called the split-field test.
The split-field test consists of double-exposing a film (sandwiched between buildup sheets) to two fields, 180 degrees apart. As shown schematically in
Figure 17.6, a square field is first exposed from above with half the field (region 2) blocked and then exposed from below to expose region 2 with region 1 blocked. Relative shift of the two images is indicative of the misalignment of the parallel opposed beams. A similar test can be performed between any two beams rotated through 180 degrees.
If beam misalignment is shown by the above test, one can then proceed to investigate the cause of misalignment by checking individually the alignment of the radiation isocenter with the axis of the collimator or gantry rotation as described previously.