Sampling (Pre-analytical phase)
1. Measure the amount of tissue sampled (individual core length, aggregate core length, number of fragments, number of cores collected, and identification of extraprostatic tissue)
2. Improve accuracy of cancer localization (e.g., imaging, 3D mapping)
3. Compare cancer yield with other urologists (? Too many or too few biopsies; electronic cancer registry reporting)
Processing (Pre-analytical and Analytical phases)
4. Implement patient biopsy identification system (bar codes or RFID)
5. Compare histotechnologist performance measures (e.g., histotechnologist’s skill in processing and cutting prostate biopsies, number of needle cores embedded per cassette, and number of tissue cuts obtained per specimen)
Reporting (Post-analytical Phase)
6. Review prior negative slides upon diagnosis of malignancy
7. Review positive slides from outside institutions
8. Pathologist skill in biopsy interpretation
9. Compare laboratory performance measures with national benchmarks
10. Use practice protocols and reporting templates (e.g., Cancer checklists of the CAP)
Prostate Biopsy Tissue Sampling (Pre-analytical phase)
QA Factor #1: Measure the Amount of Tissue Obtained to Decrease Variance
The yield of prostate cancer by biopsy is influenced by the amount of tissue collected. For example, in the USA, the biopsy side-notch instrument (e.g., Bard, Covington, GA) is used to take biopsy specimens from 10 to 12 sites. However, in other countries, some practitioners use an end-cut biopsy instrument (e.g., BioPince, Amedic, Sollentuna, Sweden) to take 8-core biopsies [3]. Core length may also be affected by the anatomic site sampled as well as the processing method used in the laboratory (see below) [4–6]. Core length of greater than 10 mm is considered by some authors to be the threshold of satisfactory quality for needle biopsies, and shorter biopsies may compromise accurate histological evaluation [4, 5]. Two recent studies found that the mean length of biopsy cores was 12.8 ± 3.5 mm in the USA and 14.1 ± 4.4 mm in Europe [4, 5].
Iczkowski and colleagues were the first to report on cancer yield as a function of biopsy tissue sampled [4]. Their study of sextant transrectal biopsies from two medical practices revealed a 3.6-fold variance in the length of tissue of single cores, with a trend for prediction of cancer yield, especially at the apex where the cores were shortest. Mean total tissue lengths sampled were 108 ± 27 mm (range 30–275) and 81 ± 22 mm (range 30–228) in each of two urology group practices. They concluded that the amount of tissue sampled by needle biopsy represents an important quality assurance consideration worthy of comparison with national standards.
Mondet and colleagues undertook a prospective study of 339 consecutive ten-core extended standardized biopsies performed by two urologists over a period of 22 months [7]. Measures of biopsy quality included mean length, amount with identified capsular or periprostatic tissue, and mean number of fragments. Initially, there were significant differences noted, but a progressive decline in variance and improvement in quality occurred as the urologists reviewed feedback on their performance. The authors concluded that systematic scoring of biopsy quality prompted the urologists to improve their practices.
A study from Verona, Italy, of 509 consecutive 14-core transperineal biopsies revealed mean length of 14.14 ± 4.35 mm; all cores were longer than 10 mm [5]. Mean length did not correlate with patient age, PSA concentration, digital rectal examination findings, or prostate volume. The percentage of fragmented cores and the rate of cores without prostatic tissue were 3% and 0.4%, respectively, significantly lower than results reported with transrectal biopsies [6].
A recent report from Reis and colleagues analyzed the incidence of core fragmentation and found that the number of core fragments obtained by biopsy was 21.54 (±3.56) compared to 24.08 (±4.77) examined by the pathologist [8]. They concluded that core fragmentation may adversely affect stage and grade consideration.
Urologist skill and standardization of collection and processing of biopsies significantly reduced variance in prostate biopsy quality in the REDUCE clinical trial (prevention of prostate cancer by dutasteride), thereby optimizing cancer detection and yield [9]. Biopsy quality was found to be a useful comparative measure in urologic practice, and the authors concluded that this should be part of all urologists’ quality assurance program. They compared biopsy quality among study sites worldwide and found significant differences between geographic regions in three measures of quality (aggregate core length, number of cores obtained, and mean length of individual cores). For example, entry biopsies from Australia contained 60% more tissue than those from Central/Eastern Europe. Also, there was a 1.2-fold difference between South America and Central/Eastern Europe in number of cores and a 1.6-fold difference between Australia and Africa in mean length of individual cores at entry. In an attempt to decrease variance in biopsy quality, they instituted a uniform 10-core biopsy collection at year 2 and trained investigators to standardize the biopsy procedure so that data would be comparable and valid in determining the efficacy of dutasteride for preventing prostate cancer. Biopsies obtained after this protocol-required standardization and investigator training showed a significant increase in all measures of biopsy quality when compared with entry biopsies, with less variance (greater uniformity) among all regions. Even the region with the greatest amount of tissue at entry (Australia) benefitted, with an increase of 24% at year 2. However, there was still a 1.1-fold difference (South America and Africa vs. Australia) in aggregate length and a 1.1-fold difference (South America vs. Australia) in mean length of individual cores at year 2, despite efforts at standardization and collection of the same number of cores.
QA Factor #2: Improve Accuracy of Cancer Localization (e.g., Imaging, 3D Mapping)
A serious unresolved issue confronting advocates of active surveillance and focal therapy is determination of the location(s) of cancer in the prostate; the corollary to this problem is determination of the extent or volume of cancer. Prostatic imaging continues to improve, but is still considered suboptimal for individual patients, and is beyond the scope of this report. Three-dimensional mapping biopsy has been proposed as a surrogate.
Earlier studies compared number and location of biopsies with cancer yield, with varying results. Eskew et al. [10] developed a 5-region, 13-core biopsy strategy which improved the cancer detection rate by 37%. However, this 13-core biopsy was associated with increased complications, such as gross hematuria. Presti et al. [11] developed a 10-core biopsy strategy including sextant biopsies and biopsies from the lateral mid and lateral base from both right and left sides, increasing cancer detection by 16% when compared with sextant biopsy. Subsequently, Presti et al. [12] demonstrated that a 12-core biopsy scheme slightly improved the cancer detection rate compared to a 10-core scheme. These and results from many other reports have resulted in migration from sextant biopsy to more extended biopsy schemes (10 or more biopsies) by most urologists in the USA.
Mapping of biopsies is critical for management and treatment of prostate cancer, but localization of the cores is often inaccurate and prone to variance in practice. Mozer et al. used a registration algorithm to represent the location of biopsies in a reference three-dimensional ultrasonographic volume in planning TRUS biopsies [13]. Overall, there was 71% success in hitting the planned targets, with substantial variability that depended on their location (100% success rate in the middle and right parasagittal prostate vs. 53% in the left lateral base). Substantial improvement was observed as the operator received feedback from the system, resulting in median biopsy tissue length improvement from 90 mm to 121 mm.
Scattoni et al. found that the most beneficial biopsy scheme varied according to the clinical characteristics of the patients [14]. They detected prostate cancer in 47% of patients with 24-core biopsies, and found that 16 cores was optimal for those with negative digital rectal examination, prostate volume of 60 cm3, and age 65 years, whereas 14 cores was most advantageous for those with a negative rectal examination, volume of 60 cm3 or >60 cm3, and age > 65 years or a negative rectal and PV > 60 cm3. They concluded that 10-core sampling permitted detection of 95% of cancers in patients with a positive rectal examination. It should be noted that this and other biopsy-only studies underestimate the true likelihood of cancer owing to the inability to examine the entire prostate gland.
In the past decade, Onik and Barzell introduced the transperineal three-dimensional mapping biopsy as an additional staging procedure prior to focal prostate cancer therapy [15, 16]. Samples were taken every 5 mm throughout the volume of the prostate using a brachytherapy grid, and each sample was labeled separately as to its grid location. In an early report of 110 patients, all of whom had unilateral cancer on transrectal ultrasound biopsies, 55% were restaged with bilateral cancer; median number of cores taken was 46 (SD ± 19). In addition, Gleason score increased in 23% of patients. Complications were self-limited. In a subsequent and remarkably similar report, Onik and colleagues studied 180 additional patients with unilateral cancer and restaged 61% with bilateral cancer; Gleason score increased in 23% [17]. Interestingly, 36 patients had negative results from 3D mapping. Subsequent reports have endorsed 3D mapping biopsies to optimize risk stratification for individual patients being considered for focal therapy [13, 18–25].
QA Factor #3: Compare Cancer Yield with Other Urologists (Too Many or Too Few Biopsies?)
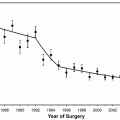
Are urologists performing too few or too many prostate biopsies? Who decides what the optimum yield of prostate cancer should be? These and other practice parameters should be included in a complete quality assurance program in urology, yet are often lacking. Such thresholds should be established by local, regional, and national benchmarking to decrease variance in practice. A Mayo Clinic study of all men in Olmstead County, Minnesota, with a first prostate biopsy performed between 1986 and 1997 revealed that the overall cancer yield of 36% was essentially unchanged across periods (p = 0.6); however, by age, cancer yield decreased from 29 to 21% (1980–1986 vs. 1993–1997) for men aged 50–59 years but increased from 38 to 45% for those aged 70–79 years [26]. The SEER database in the PSA era (through 2001) revealed an overall cancer yield of 32%; the yield increased with age (26% for men aged 65–69 years, 31% for men aged 70–74 years, 35% for men aged 75–79 years, and 41% for men aged 80 years and older) [27].
According to a European Uropathology consensus group, the frequency of atypical small acinar proliferation (ASAP) suspicious for but not diagnostic of malignancy should be less than 3% (good) or less than 5% (fair) [28]. They also suggested a threshold of less than 3% false negative diagnoses of prostate cancer after review, but we disagree, and believe that the false negative rate should be 0%. What urologist wants to be told that 3 of every 100 patients initially reported to have benign findings on biopsy actually had cancer?
Our laboratory maintains an online comparative analysis of prostate biopsy diagnoses for clients that is automatically updated daily [29]. This allows the individual urologist to compare his/her results with themselves or others (de-identified national data from our other clients), stratified by diagnosis (Table 11.2). For Dr. X (de-identified), the findings reveal that his cancer yield rate was consistently below the national database, suggesting that he may be more aggressive in undertaking biopsies than other colleagues. These data are limited and cannot account for practice differences in patient selection, demographics, etc., so they must be interpreted with caution.
Table 11.2
Comparison of prostate biopsy results of a single physician (Dr. X; n = 400 cases) with Bostwick Laboratories/National database (n = 52,480 cases)
Time period | Dr. X-benign | National database-benign | Dr. X-suspiciousa | National database-suspiciousa | Dr. X-cancer | National database-cancer |
|---|---|---|---|---|---|---|
Jul–Sep 2010 | 64.6 | 54.9 | 9.8 | 9.2 | 25.6 | 35.9 |
Oct–Dec 2010 | 63.8 | 56.6 | 7.5 | 8.4 | 28.7 | 35.0 |
Jan–Mar 2011 | 68.6 | 55.0 | 6.7 | 9.3 | 24.7 | 35.7 |
Apr–Jun 2011 | 67.2 | 55.3 | 5.0 | 9.2 | 27.8 | 35.5 |
Processing (Pre-analytical and Analytical Phases)
QA Factor #4: Implement Patient Biopsy Identification System (Bar Codes or RFID)
Patient specimen switching is a common and avoidable problem, involving about 0.5% of cases [30], and may occur at any step of the workflow process in the urology clinic and pathology laboratory. The potential for patient harm is especially high in diagnostic anatomic pathology given the impact on care by each definitive diagnosis; the most significant resulting damage is to the patient who receives an erroneous diagnosis and potentially irreversible treatment (or the lack thereof) as a result. To protect patients from errors, quality control initiatives must consider every step of this process, regardless of whether the error was caused by a clinician or nurse, laboratory professional, or a non-laboratory provider. Such steps for patient identification errors include the pre-analytical phase in the clinician’s office during which the biopsy is taken; the analytical phase in the laboratory during which the tissue is received, processed, and diagnosed; and the post-analytical phase in the laboratory and elsewhere during which the diagnostic report is delivered and the pathology slides and cassettes are stored. The recent Laboratory National Patient Safety Accreditation Program of the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) required that each laboratory “…establishes processes to maintain specimen identity throughout the pre-analytical, analytical, and post-analytical processes”[31]. Process improvement methods include the use of 2-dimensional bar codes and radiofrequency identification (RFID) tags (Bostwick DG, personal communication). The potential for biopsy mismatches in clinical practice is an under-recognized problem that requires rigorous attention to details of chain of custody and consideration of more widespread DNA identity testing.
QA Factor #5: Compare Histotechnologist Performance Measures (e.g., Histotechnologist’s Skill in Processing and Cutting Prostate Biopsies, Number of Needle Cores Embedded per Cassette, and Number of Tissue Cuts Obtained per Specimen)
There is variation between laboratories in the number of serial sections obtained from prostate tissue blocks for routine examination; we routinely obtain six sections on each of two slides, yielding a total of 12 sections [2]. The first three sections and last three sections are placed on one slide and submitted for routine hematoxylin and eosin staining; the intervening six sections are placed on another slide and saved for additional stains or special studies such as immunohistochemistry or digital image analysis for DNA ploidy analysis. In our experience, recutting the block for additional levels is useful in about half of cases, with usually no more than four additional slides before the tissue specimen is exhausted. Most biopsy specimens consist only of tissue from the peripheral zone, seldom including the central or transition zones unless the operator has specifically targeted those locations.
Prostate biopsies are particularly difficult to embed and cut because they are small and tend to fragment and curve. Flat embedding of the biopsy cores enhances the amount of tissue that is examined by the pathologist. Laboratories that process prostate biopsies with other tissues of differing density and consistency (for example, breast biopsies with abundant fatty tissue) usually handle all specimens the same way, which often results in prostate biopsies that are overstained or too thick to interpret. Similarly, overstained sections (the most common problem in our consultation practice) contain obscured nuclear chromatin without recognizable nucleoli. These problems are compounded in biopsies with small foci that are suspicious for malignancy.
Multiple needle biopsies submitted in one or two containers tend to entangle and fragment and may be difficult to embed in a single plane during processing unless the histotechnologist is experienced and careful. The resulting loss of tissue surface area makes a definitive diagnosis difficult in many cases, and results in equivocal pathology reports. If multiple cores are embedded in one cassette, all must be separated from each other. We recently compared single core vs. 3-core embedding per block, and found that the yield of suspicious foci (PIN and ASAP) and cancer was identical, indicating that vials can be thoughtfully combined without compromising patient care; different colored inks are used to differentiate the sites (Bostwick DG, Kahane H, 2012, personal communication).
Reporting (Post-analytical Phase)
QA Factor #6: Review Prior Negative Slides Upon Diagnosis of Malignancy
Cytopathologists in the USA are required to routinely review all previously negative cervical pap smears received within five years prior to the new diagnosis of high-grade squamous intraepithelial lesion or gynecologic malignancy. Patel and Layfield applied this standard to all prior negative prostatic needle biopsies following a new diagnosis of prostatic adenocarcinoma; in a 5-year retrospective study, they found a false negative rate of 0.68% (2 of 87 cases initially diagnoses as benign that contained diagnostic foci of cancer) [32




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree