CHAPTER 13 Radiation Therapy Effects and Considerations
For more than 100 years, the treatment of breast cancer was exclusively surgical. The paradigm of breast malignancy management was eventually challenged by several landmark breast preservation trials, which led to the wide acceptance of treatment of most stage I and II breast cancer patients with whole-breast irradiation following tumor excision. In 1973, in Milan, Italy, women with clinically localized breast carcinoma were randomly assigned treatment with total mastectomy or breast conservation therapy (BCT) with partial mastectomy (quadrantectomy) and whole-breast irradiation. By 1980, a total of 701 patients were randomized and treated. In the United States, a similar trial was initiated in 1976. The National Surgical Adjuvant Breast Project B-06 (NSABP B-06) randomized 2163 women with T1–T2 and N0–N1 breast cancer to one of three treatments: total mastectomy, segmental mastectomy, or segmental mastectomy and radiation therapy (RT). In 2002, the results of both studies, with 20 years of follow-up, were published in the New England Journal of Medicine. The results of both trials supported the use of BCT as equally effective compared to mastectomy, in that disease-free survival and overall survival were not adversely affected by preserving the breast. The importance of the status of margins was stressed in NSABP B-06. These two studies and many subsequent international and multi-institutional trials led to a consensus statement by the National Cancer Institute in June 1990 recommending BCT as preferable to mastectomy in appropriately selected patients.1–5
BREAST PRESERVING WHOLE-BREAST IRRADIATION (THREE-DIMENSIONAL NON-INTENSITY–MODULATED RADIATION THERAPY OR FORWARD PLANNING TECHNIQUE)
The patient is examined at consultation, paying special attention to the patient’s breast size, location of the tumor, and any wound issues. Pathology and imaging studies are reviewed. Patients requiring chemotherapy often receive a full course of systemic treatment before radiation. Before RT starts, a mammogram of the involved breast may be obtained. This will serve as a baseline study for follow-up mammograms. Case 1 in this chapter is an illustration of the potential utility of preradiation mammography. Questionable abnormalities need to be worked up before initiation of RT.
Treatment Planning
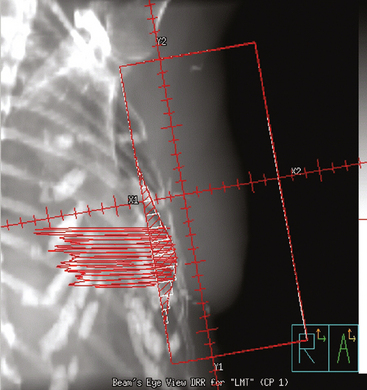
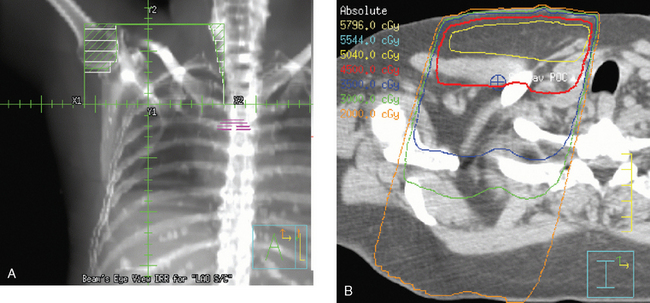
The computerized treatment planning process starts with the radiation oncologist outlining the volumes of interest (tumor bed, heart, lungs). The physicist or dosimetrist then creates an optimal arrangement of the tangential fields to cover the breast with a uniform isodose distribution (Figure 1). The dose-volume histograms are created, and the plan is reviewed and approved by the radiation oncologist. The patient is brought in for port films (Figure 2) and visual check of the fields on the skin to ensure a reproducible setup.
Tumor Bed Boost
Studies have shown that a boost dose of about 1600 cGy to the tumor bed improves local control. Radiation oncologists use the findings on physical examination, as well as pretreatment imaging studies, and correlate these with the pathology and operative reports to design the volume of tissue to be included in the boost. The patient is placed on a simulation table in a position to even out the surface of the skin over the tumor bed. The volume is outlined and marked with a wire (Figure 3). Ultrasound or CT is obtained to verify the coverage of the tumor bed and to calculate the energy of the electron beam necessary to adequately treat the surgical bed. Once the coverage is approved by the radiation oncologist, a custom lead alloy cut-out is created. The optimal energy of the electron beam is selected to adequately cover the depth of the postsurgical bed, without extending into underlying structures.6–8
WHOLE-BREAST IRRADIATION USING INTENSITY-MODULATED RADIATION THERAPY (IMRT) OR FORWARD TREATMENT PLANNING TECHNIQUE
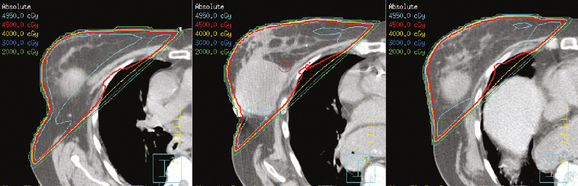
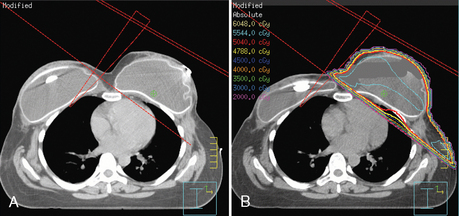
The continuing search for improved delivery of the conformal dose of radiation to the breast, while protecting the underlying structures and minimizing exposure of the contralateral breast, led to development of treatment planning algorithms using IMRT. Based purely on dosimetry, the superiority of treatment plans created using IMRT or forward planning technique (without wedges, using multiple-shaped fields) over threedimensional conformal opposed tangential fields (using wedges to compensate for uneven breast contours) is readily evident (Figure 4). The dose to the breast is more uniform, there is less of a “hot spot,” and the isodose curves are concave, conforming to the shape of the underlying chest wall. The dose to the ipsilateral lung can thus be reduced, and in cases of left breast treatment, the heart can be spared to a greater extent.
There are challenges associated with implementation of a breast cancer IMRT program on a routine basis. The planning and quality assurance processes are extensive, and the treatment time is longer (not an insignificant factor in a busy department). The patients tolerate treatment better, with less acute skin toxicity. Reduction in long-term toxicity is expected. Further efforts to improve the therapeutic index of RT (optimal dosing to the target volume and maximal protection of the surrounding tissues) involve alternative patient setup (prone), breath-hold and deep inspiration technique, and use of different fractionation. To further spare the surrounding tissues, techniques of partial-breast RT using IMRT have been developed. Partial-breast irradiation is being tested against whole-breast treatment in the ongoing NSABP B-39/Radiation Therapy Oncology Group (RTOG) 0413 study.9–11
TREATMENT OF LYMPH NODES
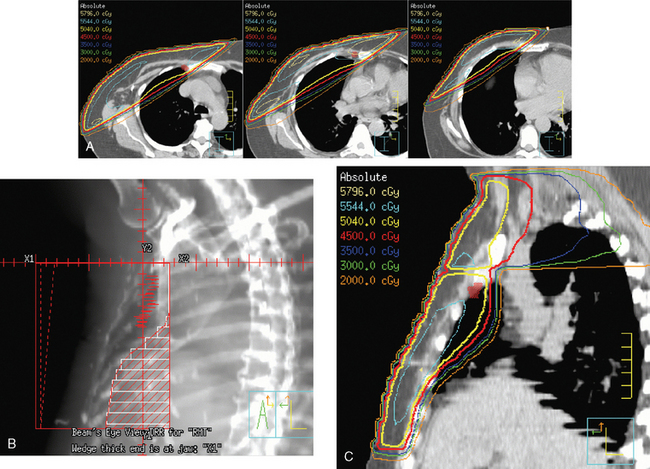
The radiation of axillary lymph nodes can be achieved with either inclusion of the level I and II lymph nodes in the tangential fields (if the CT simulation confirms good coverage and if the lung and heart can be spared) or use of a separate oblique field matched inferiorly to the upper border of the tangential breast fields. Depending on the patient’s anatomy, the use of an additional posterior axillary boost (PAB) field may be required. Coverage of the supraclavicular region depends on the available information delineating the exact location of the nodal basin and the brachial plexus (Figure 5).12–14
Internal Mammary Lymph Node Radiation Therapy
Primary lymphatic drainage into the internal mammary (IM) lymph nodes may occur in 17% to 25% of tumors located in medial, 29% in central, and 27% in lower outer quadrants of the breast. Information from lymphoscintigraphy or positron emission tomography (PET), CT, or MRI, if available, can help in localization of IM lymph nodes. Treatment of IM lymph nodes is individualized and dependent on many factors (Figure 6). Central, medial, or lower outer quadrant breast tumors with multiple positive axillary lymph nodes may require treatment of the IM lymph nodes. However, isolated IM failure is uncommon. The most commonly involved IM lymph nodes are in the second, third, and first interspaces, in that order. The potential for increased cardiopulmonary toxicity warrants caution. Published treatment guidelines give no definitive recommendation for routine treatment of IM lymph nodes and leave this decision to the discretion of the treating radiation oncologist.15,16
POST MASTECTOMY RADIATION THERAPY
Currently, postmastectomy RT is indicated in patients with large tumors (≥5 cm), involvement of four lymph nodes or more, close or positive margins, and sometimes multicentric tumors. A Danish study reported in the New England Journal of Medicine in 1997 on results of a trial in which 1708 women with stage II to III breast cancer were treated with mastectomy and then randomized to either eight cycles of Cytoxan, methotrexate, 5-fluorouracil (CMF) chemotherapy and chest wall and regional lymphatic RT, or nine cycles of CMF chemotherapy and no RT. After 114 months of median follow-up, the locoregional failure rate was 9% in the arm treated with RT, compared with 32% without RT. The 10-year overall survival rates were 54% and 45%, respectively. A Canadian study, also reported in the New England Journal of Medicine in 1997, reported results of a trial of 318 premenopausal node-positive patients after mastectomy who were randomized to undergo either CMF chemotherapy and RT or CMF chemotherapy alone. The arm treated with chemotherapy and RT received three cycles of CMF, followed by RT, followed by another three cycles of CMF. After 150 months of median follow-up, there was a 33% reduction in recurrences, a 17% improvement in disease-free survival, and a 29% reduction in breast cancer–related mortality.
As the use of postmastectomy RT has increased, so have concerns regarding the effect of RT on reconstructive tissue, implants, and the overall cosmetic treatment outcome. The Therapeutic Index of Loco/Regional Treatment must first account for optimal tumor control, while resulting in the best cosmetic outcome. It is not just cosmesis that is at stake. Development of implant encapsulation can be painful. Fat necrosis can be difficult to differentiate from tumor recurrence and often leads to biopsy, causing patients anxiety. Development of infection or seromas in the reconstructed breast may lead to the removal of transplanted or implanted tissue. Although all these adverse outcomes can occur without use of RT, the incidence does increase with exposure of transplanted tissue or implanted devices to radiation. It is thus extremely important that any patient with even a remote possibility of needing postmastectomy RT be thoroughly evaluated by the whole team to select the best surgical option.17–21
POSTMASTECTOMY RADIATION AND BREAST RECONSTRUCTION
Before mastectomy, patients should undergo an extensive consultation with review of the available reconstructive options. Clinically staged patients who are not expected to require postmastectomy RT are excellent candidates for immediate reconstruction. There are many factors that need to be taken into account while selecting the best surgical reconstruction option. Tumor-related considerations include the tumor size and location, multifocality or multicentricity, lymph node involvement, and chest wall fixation. Patient-related factors include body habitus, medical conditions affecting tissue healing (e.g., collagen vascular diseases), prior chest RT, the location of prior breast surgery scars, smoking history, and prior or planned chemotherapy. Patients with larger tumors (>5 cm), more than three lymph nodes involved, or close margins will require postmastectomy RT. Reconstructive surgery in this population of patients has to be planned carefully. Optimal approaches and timing of reconstruction are the subjects of ongoing discussion and continuing evolution. At many institutions, the anticipated need for postmastectomy RT is a relative contraindication to immediate breast reconstruction using implants. Delayed immediate reconstruction is a viable option in management of patients at high risk for needing postmastectomy RT. During mastectomy, tissue expanders are placed. While the patient is recovering from surgery and, if applicable, undergoing chemotherapy, tissue expansion is achieved by ongoing repetitive injections of saline. This process must be completed before the simulation and radiation treatment planning process can begin (Figure 7).21
Transverse rectus abdominis myocutaneous (TRAM) flap reconstruction surgery should be delayed if possible, until after completion of the full course of radiation. This allows for an optimal cosmetic outcome22,23 and improved technical coverage with the radiation fields.24,25
LOCOREGIONAL TREATMENT OF BREAST CANCER IN THE ERA OF NEOADJUVANT CHEMOTHERAPY
In an attempt to reduce false-negative sentinel lymph node findings after neoadjuvant chemotherapy, many surgeons favor routine sentinel lymph node sampling before initiation of chemotherapy. Positive sentinel lymph node biopsy would lead to completion axillary dissection, before or after chemotherapy. Pathology from prechemotherapy sentinel lymph node biopsy is used by the radiation oncologist to plan the extent of the radiation fields.26,27
NEOADJUVANT CHEMOTHERAPY TO ATTEMPT BREAST PRESERVATION
The increasing use of neoadjuvant chemotherapy for BCT necessitates even closer cooperation of the multidisciplinary team managing these patients. Unless there are obvious microcalcifications to mark the tumor, the lesion needs to be marked with a clip either before or within the first two cycles of chemotherapy. This is usually done with ultrasound or mammography guidance. In patients who reach clinical complete remission, these clips will guide the surgeon in planning the lumpectomy and the radiation oncologist in planning the extent of the tumor bed boost.28
RADIATION THERAPY IN THE MANAGEMENT OF DUCTAL CARCINOMA IN SITU
Three randomized studies comparing tumor resection (with clear margins) with or without RT reported a strong beneficial effect of RT on local recurrence rates, but no effect on overall survival. Chemoprevention with tamoxifen further protects the involved, as well as the contralateral, breast. The technique of RT is the same as that for invasive tumors. There is no need for treatment of lymph nodes, although sentinel lymph node biopsy may be indicated in high-grade or large tumors.29–31
EVOLUTION OF RADIATION THERAPY IN BREAST CONSERVATION THERAPY
Rationale and Options for Partial Breast Radiation
Numerous randomized trials32–35 comparing whole-breast radiation after tumor excision to tumor excision alone have shown that most breast tumors recur in the tumor cavity. Additionally, these trials have shown that the risk for recurrence outside the tumor cavity is similar whether or not whole-breast radiation was given. This suggests that additional radiation given outside the tumor cavity may not be of additional benefit to patients.
Patients are potential candidates for accelerated partial breast RT if they have stage 0, I, or II tumors, a single tumor less than 3 cm in maximal dimension, minimal nodal involvement, and clear surgical margins. Typically, partial-breast radiation is delivered twice a day, with treatments given at least 6 hours apart, for a total of 10 fractions.
Interstitial breast brachytherapy alone has been successfully used at some U.S. centers for more than 10 years following breast-conserving surgery. A trial was started by Vicini and colleagues in 1993 using brachytherapy as the only radiation treatment modality for patients following breastconserving surgery.36,37 By 2001, 120 patients were enrolled in this trial. Four patients developed local recurrence at a median follow-up of 82 months. During 1997 to 2000, 100 patients were enrolled in an RTOG prospective phase I/II study of breast brachytherapy. At a median follow-up of 2.7 years, most patients experienced only mild treatment-related toxicities.38
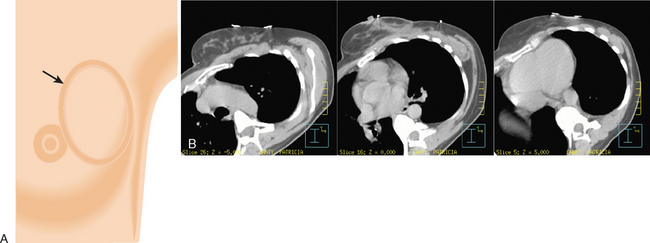
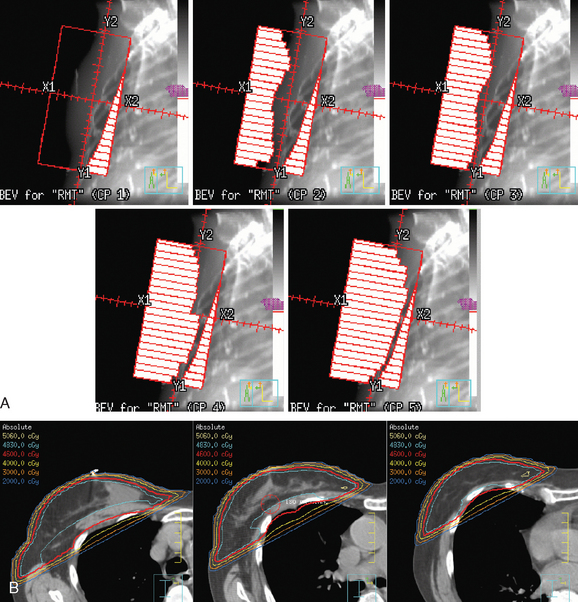
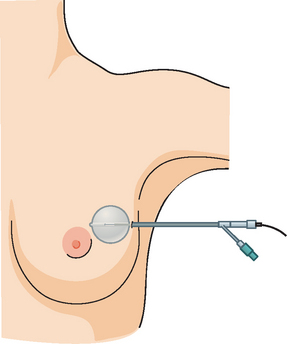
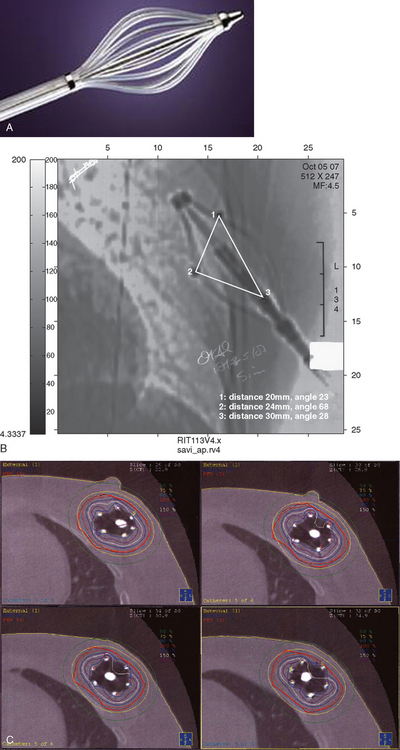
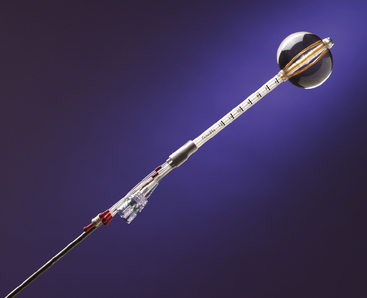
In 2002, the U.S. Food and Drug Administration approved the Proxima Therapeutics MammoSite balloon catheter for intracavitary high-dose-rate breast brachytherapy (Figure 8). Seventy patients were initially enrolled in a prospective multicenter trial evaluating the safety of the MammoSite balloon catheter. Subsequent evaluation of 43 patients eligible for the therapy revealed only mild to moderate self-limited side effects.39 Advantages of the balloon catheter are that it is easier to place in the cavity, placement is more reproducible, and patient comfort is improved. Only a single catheter needs to be temporarily placed in the lumpectomy cavity, as opposed to 10 to 20 catheters with traditional interstitial implants. However, the balloon needs to “conform” properly to the tumor cavity and optimal dosimetry could be problematic if a large air pocket develops along the periphery of the cavity. In addition, balloon catheters may not be appropriate for tumors near the skin surface. A more recent addition to the partial-breast RT armamentarium is the SAVI device, which allows a radiation oncologist to selectively direct radiation through up to 7 catheter channels, allowing more tailored manipulation of the isodose (Figure 9). The Contura multilumen balloon catheter combines features of these partial-breast RT devices, with multiple offset lumens around a balloon, also allowing dose-shaping opportunities to minimize skin and rib doses (Figure 10).
Three-dimensional conformal radiation technology has been developed and improved in recent years. This technique of accelerated partial breast radiation has the advantage of being noninvasive, eliminating an additional procedure and allowing many medical groups that do not perform brachytherapy to offer partial breast RT. No adverse side effects were seen in 28 patients treated with three-dimensional conformal radiation in a 1999 pilot study.40 A potential disadvantage is that the breast is not a stationary target, and there is the potential for a geographic miss with external RT to a small target.
In 2005, the NSABP, along with the RTOG, activated a phase III randomized trial comparing whole-breast RT and partial-breast RT in women with stage 0, I, or II breast cancer. The trial is currently open and is expected to accrue 3000 patients over a period of about 2 years and 5 months. This trial will be comparing overall survival, recurrence-free survival, distant recurrence-free survival, and quality-of-life issues in women receiving whole-breast or partial-breast RT.32–40
RADIATION AFTER AUGMENTATION
Patients who develop breast cancer after they have undergone prior breast augmentation present additional challenges in designing their treatment to achieve optimal locoregional control and the best possible cosmetic outcome. With careful dosimetry, using IMRT or three-dimensional treatment planning, breast tissue coverage with the desired isodose can be achieved, although there are technical challenges associated with the presence of the implanted device.25,41
These patients should receive an extensive review of the expected effects of radiation on the augmented breast. They need to be evaluated by the radiation oncologist, breast surgeon, and plastic surgeon (if not the same) before the definitive cancer procedure is scheduled. Often the decision whether to retain, remove, or modify the existing augmentation device is patient driven. However, the patient has to be given appropriate information so that she can make an informed decision. One of the concerns frequently voiced by patients is the effectiveness of follow-up imaging. Cases illustrating the range of postoperative appearances of a variety of reconstruction options, as well as manifestations of recurrences and treatment-related changes, are presented throughout this text, particularly in Chapter 6.41
IMAGING FOLLOW-UP OF RADIATION THERAPY–TREATED BREAST CANCER PATIENTS
BCT and RT induce readily visualized and potentially confusing changes in the treated breast on imaging, particularly on mammograms, CT scans, and breast MRI, with fewer effects generally noted on ultrasound and PET. Early postirradiation mammograms in particular can show variable degrees of skin thickening and a generalized increase in breast density and reticulation. These changes usually regress with time. In addition to routine follow-up imaging, clinically detected change should prompt imaging evaluation for possible recurrence. As with all breast imaging, mammography is the cornerstone modality for initial imaging, liberally supplemented with ultrasound. In cases in which there is persistent concern for possible recurrence, breast MRI can be very helpful, particularly more than 18 months after treatment, when little if any enhancement is seen in normal postoperative scars. Significantly enhancing scars older than 18 months should be further investigated, such as with biopsy, to exclude recurrent disease. A scar complicated by fat necrosis or fat necrosis developing in the treatment bed can at times perfectly mimic a local recurrence. Examples are presented in Chapters 6 and 7. A documented local recurrence should prompt systemic evaluation, to confirm that the recurrence is local only, before decision making on additional therapy. This is best accomplished with PET/CT and bone scan.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree