CHAPTER 10 Thoracic Metastases, Mimics, and Treatment Effects
Most breast cancer patients with intrathoracic metastases are asymptomatic, the exception being those with significant pleural effusions. Therefore, unless pleural, parenchymal, or nodal metastases are demonstrated at initial staging, thoracic metastases are most commonly found at the time of follow-up imaging. A significant number are found on chest x-rays done for other reasons. Isolated involvement of the lung or pleural space occurs in 15% to 25% of women with metastatic breast cancer.1
Pulmonary metastases are typically peripheral,2 and, if large enough, may be discovered on preoperative chest radiographs. Because of its low cost, chest radiography may be a reasonable choice as a baseline study or in follow-up; however, the extremely low yield, false-positive results, inferior sensitivity compared with CT, and lack of data supporting an outcome benefit limit routine use in follow-up.
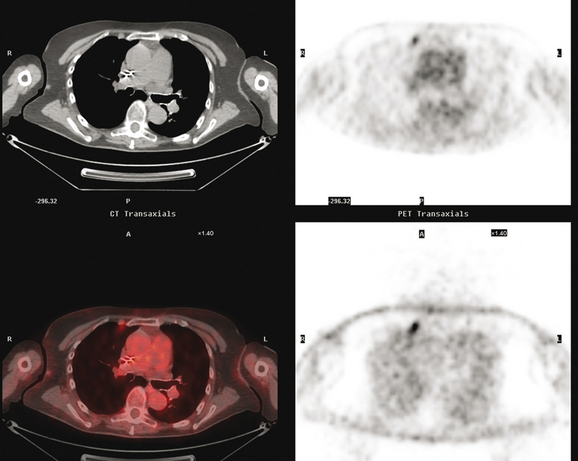
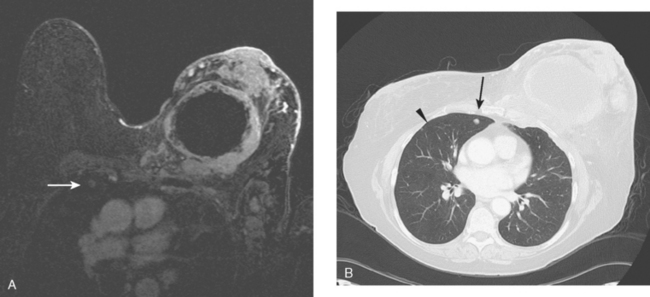
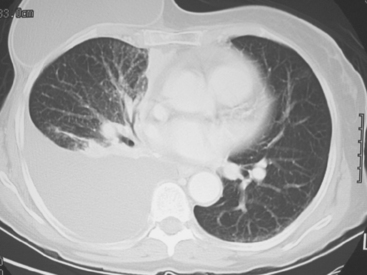
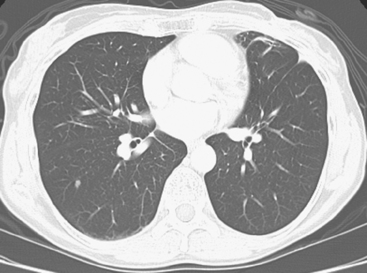
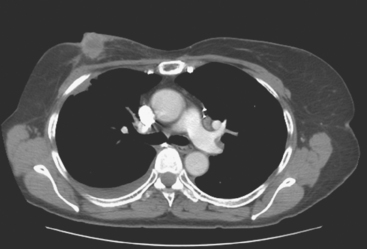
CT is the preferred modality for investigation of thoracic metastases. CT has proven efficacy for detecting local, regional, and distant disease. Normal-sized internal mammary nodes are not visualized on CT, so a patient in whom CT identifies a node or nodes larger than 6 mm is at risk for malignant involvement.3 Because of the recognized problem of relying on size only to diagnose disease, positron emission tomography (PET)/CT is presently considered the modality of choice for assessing internal mammary nodes (Figure 1). In women who die from disseminated breast cancer, intrathoracic nodal metastases may occur in more than 70% of patients.4 As with internal mammary nodes, using size as the major criterion for disease identification reduces both sensitivity and specificity. Magnetic resonance imaging (MRI) can detect most lesions larger than 5 mm, does not expose the patient to ionizing radiation, and has acceptable imaging times; however, it is generally viewed as complementary to CT, rather than as a replacement for CT (Figure 2).5–9 Cardiac and respiratory motion, reduced sensitivity for lesions smaller than 5 mm (compared with CT), calcified metastases, and suboptimal ability to assess lymphangitic spread are recognized limitations of MRI in the thorax.10
In one series of 73 patients who underwent FDG PET and CT for recurrent or metastatic disease, PET proved more sensitive (85% versus 50%) and specific (90% versus 83%) than CT. Also, PET uptake in mediastinal and internal mammary nodes was 2 times more prevalent than enlarged nodes on CT.11 FDG PET has been shown to be significantly more accurate in staging the mediastinum in patients with non–small cell lung cancer, using histology as the gold standard.12–13 Extrapolation of these data to patients with breast cancer may be problematic. It has been recognized that certain subtypes of breast cancer (e.g., invasive lobular, tubular, carcinoma in situ) show relatively low FDG uptake, resulting in reduced sensitivity compared with infiltrating ductal carcinoma (IDC) primary tumors.14–15 Theoretically, patients whose initial staging FDG PET is negative in these tumor subtypes (especially if the primary has not been excised) may demonstrate reduced sensitivity for detection of metastases, including within the chest, on restaging studies.
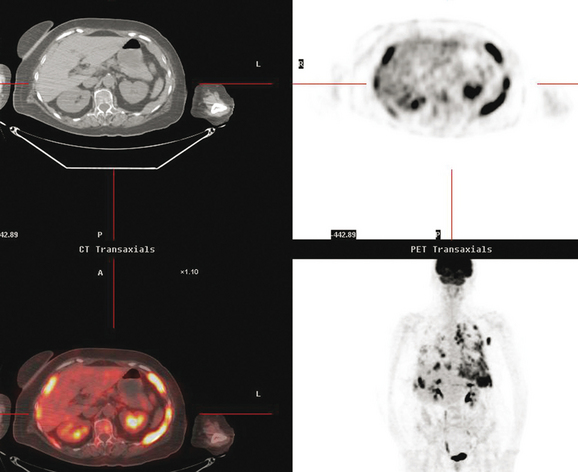
Pleural involvement may be suspected based on clinical signs on physical examination or symptoms (e.g., cough, shortness of breath, pleuritic-type chest pain, orthopnea), the discovery of a pleural effusion on plain radiography or CT, or the identification of pleural abnormalities on CT or FDG PET (Figure 3). Pleural effusion is often unilateral and on the same side as the breast primary.16 The effusion can be confirmed by chest radiography, CT, or even ultrasound. Thoracentesis may be performed for both diagnostic and therapeutic purposes. If the result is nondiagnostic, or negative with a strong clinical suspicion of malignancy, consideration may be given to FDG PET imaging (preferably, PET/CT).
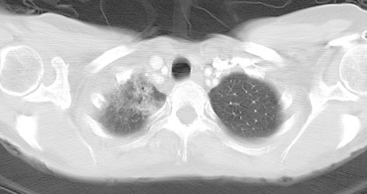
Many special circumstances exist with respect to the thoracic manifestations of breast cancer, reviewed in depth by Jung and associates.17 One important consideration is radiation therapy–related complications. Radiation pneumonitis typically occurs within 3 months of radiation therapy and progresses from diffuse haziness to coalescence of areas of consolidation to fibrous changes on chest x-ray or CT. Findings in the irradiated field may persist for months to years (Figure 4). Although PET/CT has demonstrated efficacy in separating scar from active tumor in many malignancies, its use following radiation therapy in the irradiated field may be problematic for months to 1 year or more. The heightened metabolic activity of activated monocytes at sites of radiation pneumonitis can last for a variable period of time, especially in the lung and with head and neck tumors. A negative FDG PET study at a site of postirradiation change on chest x-ray or CT is reassuring; however, a positive study must be interpreted with caution and reassessed or further investigated before initiation of additional tumor-targeted therapy.
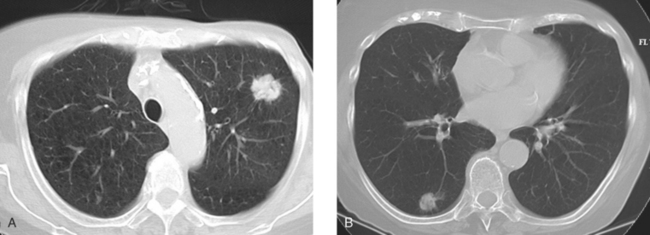
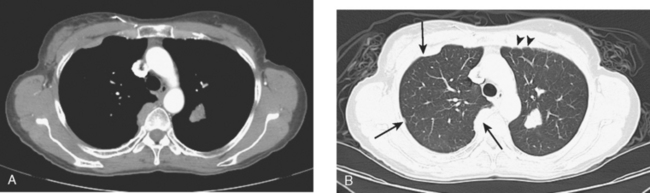
The development of a solitary pulmonary nodule in patients who have been treated for breast cancer should not be assumed to be a breast recurrence without histologic proof. About half of these patients will have lung cancer, and a small percentage of cases will be benign.18 These patients are excellent candidates for PET/CT. Hypermetabolic uptake in the nodule directs the patient to tissue sampling (sometimes obviated by the finding of widespread metastases). Absent FDG uptake, as well as an otherwise negative study, may allow watchful waiting with short-term CT follow-up (e.g., 3 to 6 months). Recall the limitations of PET with respect to histology and lesion size.
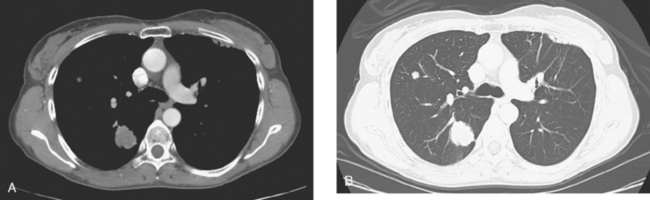
Lymphangitic metastases are extremely common in women who die from breast cancer.19 CT is the imaging modality of choice for the identification of this process, which is most often bilateral (Figure 5). Findings include irregular thickening of the interlobar septa and peribronchovascular sheaths as well as thickening of the structures in the central regions of the secondary pulmonary lobules.20
1 Patanaphan V, Salazar OM, Ricco R. Breast cancer: metastatic patterns and their prognosis. South Med J. 1998;81(9):1109-1112.
2 Shaw JP, Glassman LR. Thoracic metastases from breast cancer. In: Breast Cancer. Philadelphia: Elsevier Churchill Livingston; 2005:661-666.
3 Meyer JE, Munzenrider JE. Computed tomographic demonstration of internal mammary lymph-node metastases in patients with locally recurrent breast carcinoma. Radiology. 1981;139:661-663. (A)
4 Thomas JM, Redding WH, Sloane JP. The spread of breast cancer: importance of intrathoracic lymphatic route and its relevance to treatment. Br J Cancer. 1979;40:540-547.
5 Feirerstein IM, Jicha DL, Pass HL, et al. Pulmonary metastases: MR imaging with surgical correlation—a prospective study. Radiology. 1992;181:123-129.
6 Müller NL, Gamsu G, Webb WR. Pulmonary nodules: detection using magnetic resonance and computed tomography. Radiology. 1985;155:687-690.
7 Panicek DM. MR imaging for pulmonary metastases? Radiology. 1992;182:10-11.
8 Webb WR, Sustman HD. MR imaging of thoracic disease: clinical uses. Radiology. 1992;182:621-630.
9 Ohno Y, Sugimura K, Hatabu H. MR imaging of lung cancer. Eur J Radiol. 2002;44(3):172-181.
10 Vogt FM, Herborn CU, Hunold P, et al. HASTE MRI versus chest radiography in the detection of pulmonary nodules: comparison with MDCT. AJR Am J Roentgenol. 2004;183:71-78.
11 Eubank WB, Mankoff DA, Takasugi J, et al. 18Fluorodeoxyglucose positron emission tomography to detect mediastinal or internal mammary metastases in breast cancer. J Clin Oncol. 2001;19:3516-3523.
12 Vansteenkiste JF, Stroobants SG, De Leyn PR, et al. Lymph node staging in non-small-cell lung cancer with FDG-PET scan: a prospective study on 690 lymph node stations from 68 patients. J Clin Oncol. 1998;16(6):2142-2149.
13 Scott WJ, Gobar LS, Terry JD, et al. Mediastinal lymph node staging of non-small-cell lung cancer: a prospective comparison of computer tomography and positron emission tomography. J Thorac Cardiovasc Surg. 1996;111(3):642-648.
14 Crippa F, Seregni E, Agresti R, et al. Association between [18F]-fluorodeoxyglucose uptake and postoperative histology, hormone receptor status, thymidine labeling index and p53 in primary breast cancer: a preliminary observation. Eur J Nucl Med. 1998;25:1429-1434.
15 Avril N, Menzel M, Dose J, et al. Glucose metabolism of breast cancer assessed by 18F-FDG PET: histologic and immunohistochemical tissue analysis. J Nucl Med. 2001;42:9-16.
16 Connolly JEJr, Erasmus JJ, Patz EFJr. Thoracic manifestations of breast carcinoma: metastatic disease and complications of treatment. Clin Radiol. 1999;54:487-494.
17 Jung JI, Kim HH, Park SH, et al. Thoracic manifestations of breast cancer and its therapy. RadioGraphics. 2004;24:1269-1285.
18 Casey JJ, Stempel BG, Scanlon EF, et al. The solitary pulmonary nodule in the patient with breast cancer. Surgery. 1984;96:801-805.
19 Kreisman H, Wolkove N, Finkelstein HS, et al. Breast cancer and thoracic metastases: review of 119 patients. Thorax. 1983;38:175-179.
20 Webb WR, Müller NL, Naidich NP. High-Resolution CT of the Lung, 3rd ed. Philadelphia: Lippincott Williams & Wilkins, 2001.
CASE 1 Solitary pulmonary nodule in the breast cancer patient: Primary lung cancer versus breast cancer metastasis
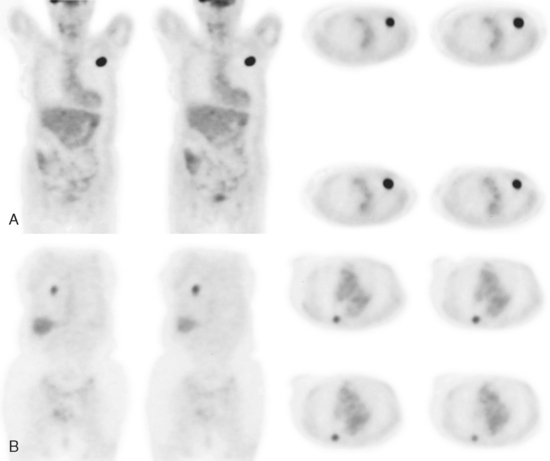
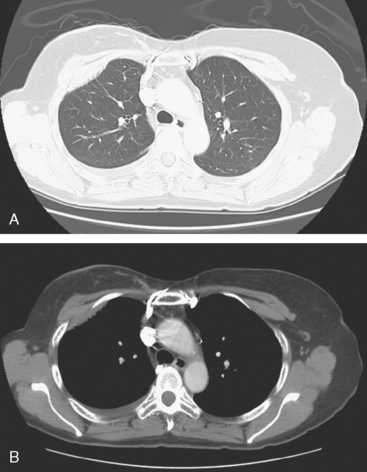
An 80-year-old woman with emphysema was found to have a left upper lobe (LUL) mass on chest x-ray. This was confirmed on chest CT, which also showed a similar-appearing, suspicious right lower lobe (RLL) lesion (Figure 1). Fine-needle aspiration (FNA) of the LUL lesion was consistent with a non–small cell lung carcinoma. The patient’s past medical history was significant for prior left mastectomy for breast cancer nearly 20 years earlier. Lymph nodes were reportedly negative, and the patient did not undergo additional therapy at that time. She also had a significant past smoking history, on the order of 1.5 packs per day.
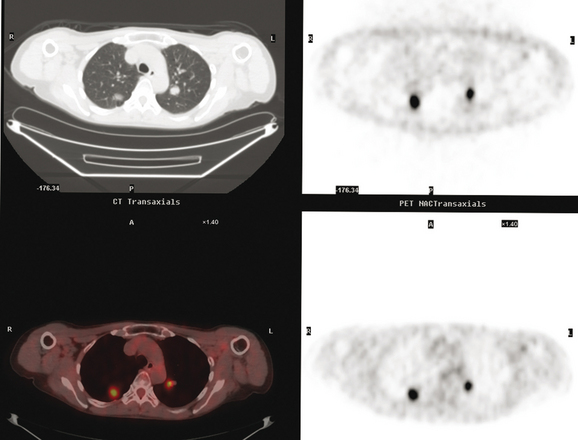
The PET scan showed both the LUL and RLL lesions to be hypermetabolic (Figure 2). Based on the information available, this result seemed most consistent with synchronous lung cancers. However, subsequent immunocytochemical stains performed on the material obtained from FNA suggested the cause to be metastatic breast carcinoma.
CASE 2 Lung metastases, progression to pleural metastases
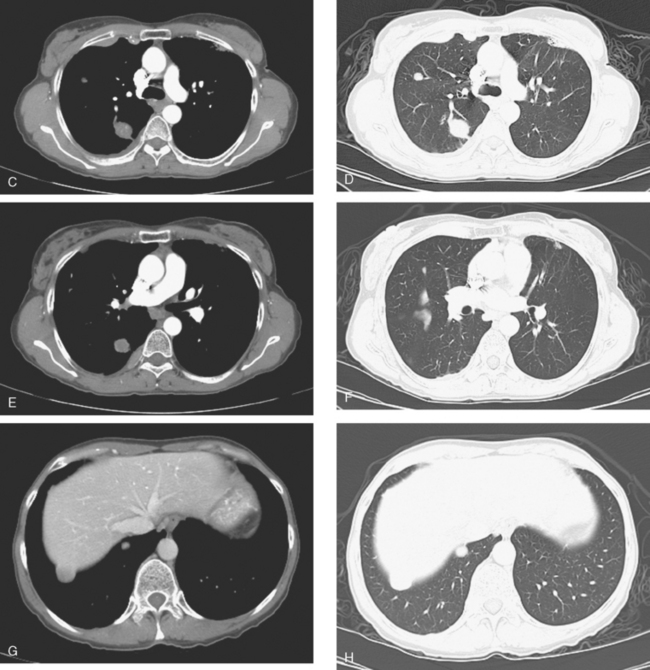
At the time of restaging, the patient was essentially asymptomatic. Chest CT and positron emission tomography (PET) showed four lung nodules on CT, three of which were hypermetabolic on PET (a 3 × 7-mm nodule was probably too small) (Figures 1, 2, 3, 4, and 5).
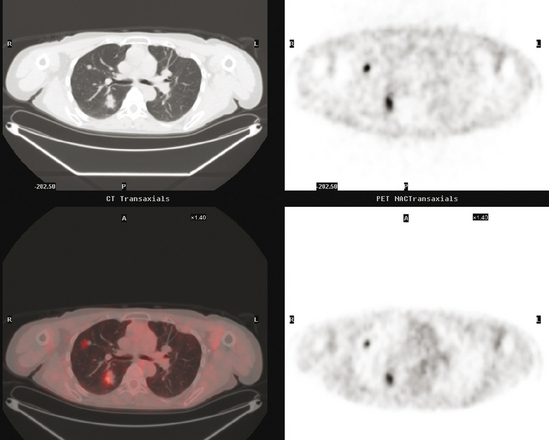
FIGURE 4 A more inferior PET/CT image shows hypermetabolism in the two right lung lesions depicted in Figure 1.
Seven months later, follow-up CT scans showed new right pleural and mediastinal disease, confirmed as fluorodeoxyglucose avid on PET (Figures 6 and 7). The patient was not particularly symptomatic, and was reluctant to have chemotherapy. She began a trial of androgenic hormone therapy (Halotestin).
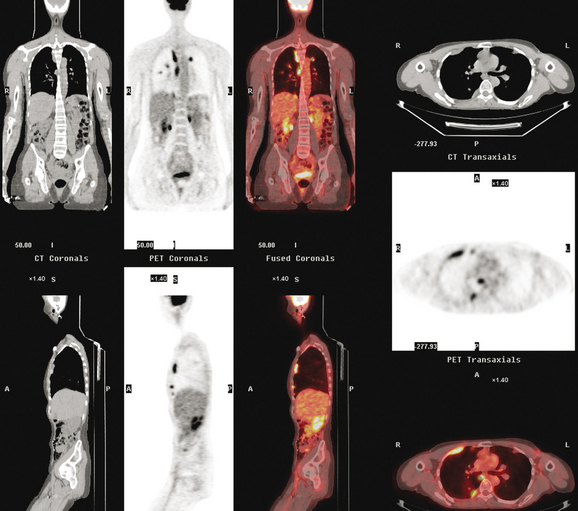
FIGURE 7 Concurrent PET/CT, 7 months after PET scan in Figures 3 to 5, shows new peripheral linear and nodular right hemithoracic hypermetabolism, which corresponds to the new pleural masses on CT and represents pleural metastases.
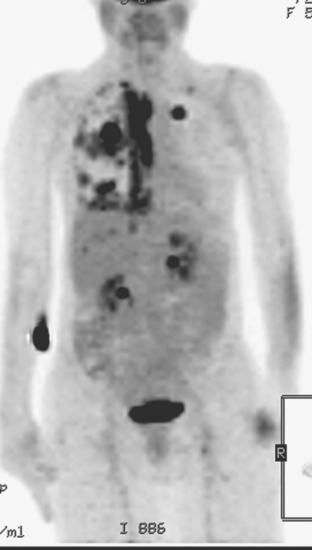
When she was re-evaluated 3 months later, she noted right posterior chest pain and dry cough. Imaging showed further progression. She was restaged in conjunction with the entrance requirements of a chemotherapy clinical trial. These studies showed progression of right pleural metastases and new liver metastases (Figure 8).
CASE 3 Chest wall, pleural, and thoracic nodal recurrence
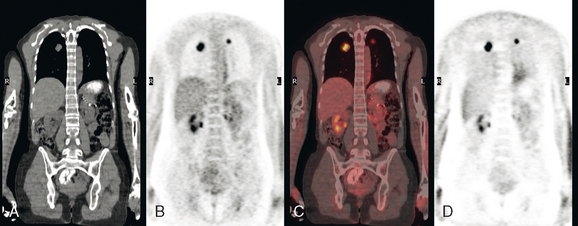
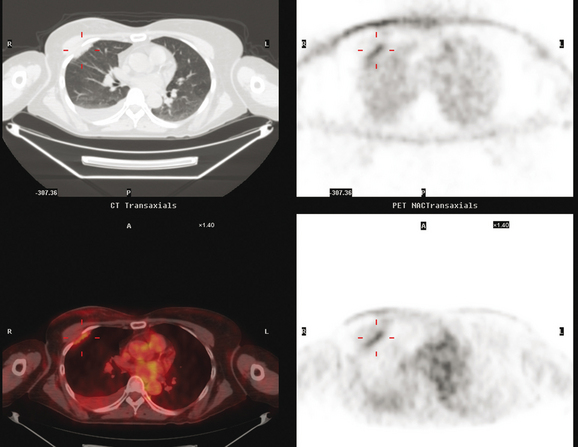
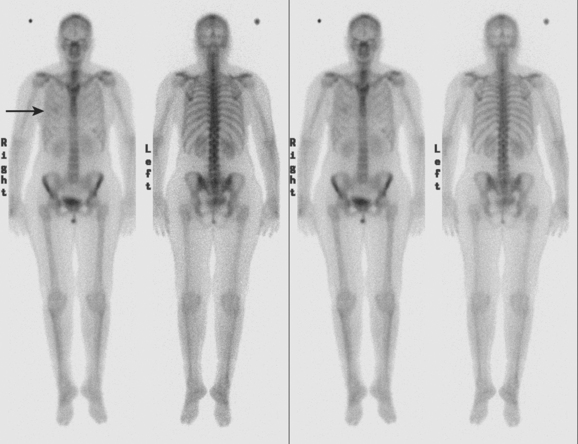
Initial evaluations suggested local recurrence only, and mastectomy was planned. However, her CA-125 was noted to be elevated, leading to restaging with positron emission tomography (PET)/CT, diagnostic enhanced body CT scans, and bone scan. PET and CT findings suggested thoracic recurrence (Figures 1, 2, 3, 4, and 5). CT scans showed a moderate-sized right pleural effusion with modest associated metabolic activity on PET. A 1-cm nodule in the right major fissure was metabolically active, as was a left hilar lymph node. The known right breast local recurrence was intensely hypermetabolic and could be faintly visualized as soft tissue activity on bone scan (Figure 6).
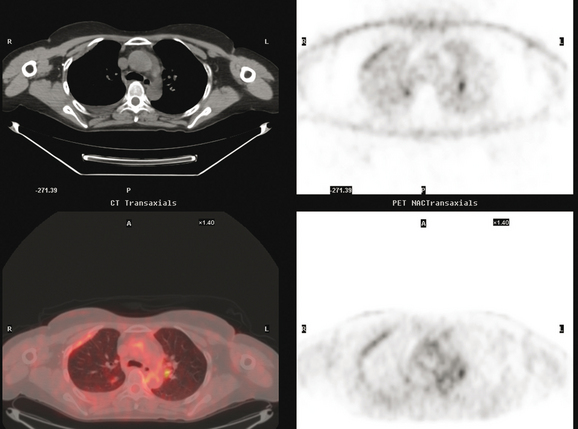
FIGURE 4 Axial PET/CT images, corresponding in level to Figure 2, show the right pleural nodules to be metabolically active. The associated activity is better seen on the NAC image (upper right) than on the AC image (lower right). A linear band of right anterolateral subpleural activity corresponds with the radiation fibrosis changes on CT, and was 8 years old. Activity corresponding with the left hilar lymph node is also seen. Note also subtle increased dependent activity in the right hemothorax compared with the left, seen on fused PET/CT (lower left) and AC PET (lower right), correlating with the pleural effusion. This, coupled with the pleural nodularity and activity, suggests a malignant etiology of the effusion, which was subsequently confirmed histologically.
Based on the PET and CT findings, CT-guided thoracentesis and FNA of the right pleural nodule were performed. Both confirmed metastatic breast cancer by morphology and immunohistochemistry. Thyroid transcription factor-1 was noted to be negative, largely eliminating lung cancer from the differential.
TEACHING POINTS
Pleural metastases are commonly manifested by the development of pleural fluid, which most often occurs on the same side as the primary breast carcinoma. The route of spread is thought to be lymphatic rather than hematogenous, which would be expected to be bilateral. This propensity for same-side laterality of pleural metastases is demonstrated in this case. The findings can range from effusion alone to fluid with variable degrees of pleural thickening and enhancement. This can be fairly smooth, as in this case, or frankly nodular and masslike (see Case 5). Activity of pleural effusions on bone scans (see Case 4) and PET scans suggests a malignant etiology. In this case, both the pleural fluid and the nodules in the major fissure showed metabolic activity on PET, a constellation of findings strongly suggesting a malignant etiology, which was subsequently confirmed histologically.
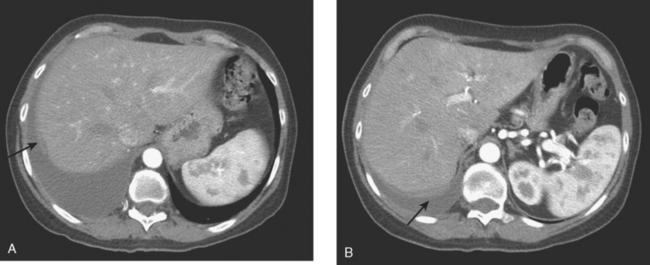
CASE 4 Pleural recurrence; bone scan and CT findings
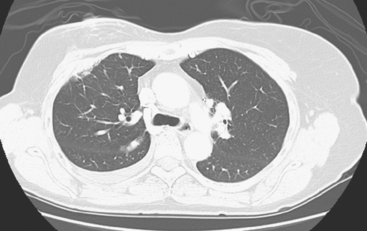
Diagnostic thoracentesis showed malignant cells consistent with breast primary, which were estrogen receptor and progesterone receptor positive. The patient was placed on anastrozole (Arimidex) after restaging studies showed no evidence of other disease (Figures 1 and 2).