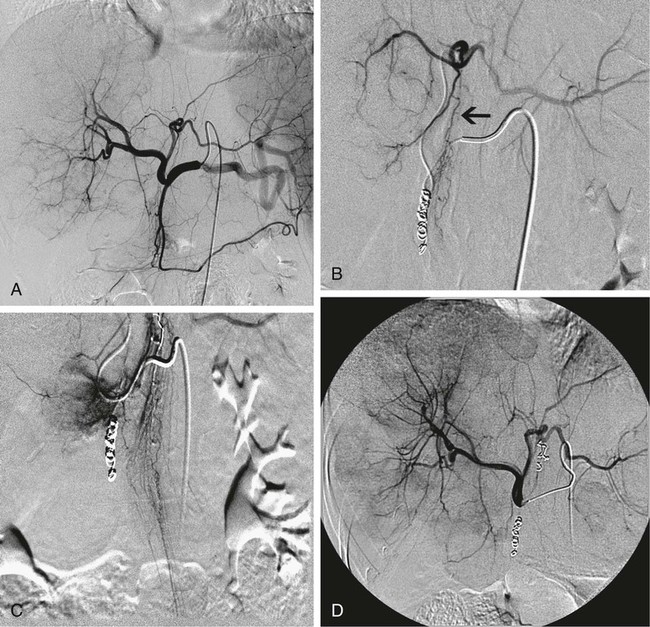
Chapter 67 Khairuddin Memon, Robert J. Lewandowski and Riad Salem Metastatic disease to the liver is the most common form of hepatic malignancy, the colon being the most common primary tumor location.1 At presentation, most of these oncology patients are not candidates for operative cure, and many have failed systemic chemotherapeutic regimens. The safety and efficacy of radioembolization with yttrium-90 (90Y) in these patients with liver-dominant disease and good performance status have been reported in the literature.2–7 Radioembolization is performed using percutaneous transarterial techniques in the interventional radiology suite. By definition, radioembolization is injection of micron-sized embolic particles loaded with a radioisotope; an interdisciplinary team is crucial to the success of a 90Y radioembolization program. This team should be well represented with members from interventional radiology, medical, radiation and surgical oncology, transplant surgery, nuclear medicine, radiation oncology, hepatology, and radiation safety. TheraSphere was approved in 1999 by the U.S. Food and Drug Administration (FDA) under a Humanitarian Device Exemption (HDE) for treatment of unresectable hepatocellular carcinoma (HCC) in patients who can have appropriately positioned hepatic arterial catheters.8 Medical professionals are directed to published FDA guidance documents on HDEs for uses in diseases other than HCC.9 SIR-Spheres was granted full premarket approval in 2002 by the FDA for treatment of colorectal cancer (CRC) metastases in conjunction with intrahepatic floxuridine (FUDR).10 Given the FDA approval for both devices, their use in disease states other than the strict indication represents the practice of medicine. In other words, use of this therapy for indications other than HCC or colorectal liver metastases is not experimental, but rather supported by ample phase 2 data. Appropriate patient selection is critical to avoid morbidity and mortality from radioembolization. Irrespective of liver tumor etiology, evaluating a patient for possible treatment with 90Y should be driven by the patient’s Eastern Cooperative Oncology Group (ECOG) performance status (Table 67-1) rather than their clinical stage. From a practical standpoint, patients with ECOG 0, 1, or 2 status (equivalent to Karnofsky score of 60% or higher) should be considered for therapy. For patients who do not fulfill this criterion (ECOG > 2), there is a relative contraindication to radioembolization. Decision to treat these patients should be based on individual patient evaluation and clinical judgment and intent. Besides performance status, prothrombin time (PT), albumin, and total bilirubin should be scrutinized because they have been shown to be the best indicators of overall liver function.11 If these values are abnormal, greater care must be taken in treating, given the relative higher risk for radiation-induced liver disease and failure. Once the patient is clinically deemed a candidate for radioembolization, angiography and 99mTc-MAA infusion are performed. These tests may reveal further contraindications to treatment, which include uncorrectable flow to the GI system and excessive pulmonary shunting.12 TABLE 67-1 The lungs can tolerate 30 Gy per treatment session and 50 Gy cumulatively.13 Therefore, when treatment planning is undertaken and lung shunting is identified, the total cumulative pulmonary dose must be calculated. Patients may be treated if their cumulative pulmonary dose will not exceed 50 Gy cumulatively for all planned infusions of 90Y. However, in patients with compromised pulmonary function, such as chronic obstructive pulmonary disease or previous lung resection, caution must be taken when approaching total cumulative doses. In these instances, the decision to treat must be individualized and based on overall clinical status. All radioembolization patients initially undergo mesenteric angiography and a 99mTc-MAA lung shunting scan.12,14–16 The angiographic evaluation required has been described by Liu et al.14 An abdominal aortogram facilitates proper visceral catheter selection as well as assesses aortic tortuosity and mural atherosclerotic disease. The superior mesenteric artery is studied to assess any variant vessels to the liver (accessory or replaced right hepatic), as well as for visualization and identification of a patent portal vein. The celiac artery is injected to study the hepatic branch anatomy. Subsequently, selective left hepatic (flow to segments 2, 3, 4A, and 4B), right hepatic (flow to segments 1 [caudate lobe may have other blood supply], 5, 6, 7, and 8), and gastroduodenal (flow to the pancreas, stomach, small bowel, and omentum) arteriograms are performed. To visualize small vessels as well as vessels that may demonstrate reversal of flow (e.g., result of flow shunt, sumping secondary to hypervascular tumor), dedicated microcatheter injection with relatively high rates (2-3 mL/s for 8-12 mL) should be performed.14 Without adequate contrast bolus, many ancillary vessels (which have profound effect on hemodynamics and directed therapy) may go unnoticed. Although it may be argued that high injection rates may represent supraphysiologic flow dynamics, the potential changes induced as a result of regional therapy with radioembolization (spasm, ischemia, stasis, and vessel injury) may result in altered physiologic states and thus reflux into these vessels. Aggressive prophylactic embolization of vessels prior to therapy is highly recommended, such that all hepaticoenteric arterial communications are completely eliminated. These vessels include the falciform, accessory or left phrenic, right or accessory gastric arteries (from the left hepatic artery), supraduodenal, retroduodenal, and accessory right hepatic artery feeding segment 6 (from gastroduodenal artery [GDA]). Reflux of microspheres into the GDA or gastric arterial circulation may result in grave clinical consequences that include severe ulceration, GI bleeding, or pancreatitis. Given the serious clinical risks of microsphere reflux into nontarget organs and the lack of clinical consequences of prophylactic embolization of the GDA/right gastric, the latter approach is strongly favored.7,14,16,17 Figure 67-1 demonstrates the principles of prophylactic embolization before radioembolization. where BSA is body surface area; or by recommended dose according to % tumor burden: • Infusions should be performed using 3F systems. This includes standard lobar infusions as well as cases with difficult anatomy, small vessels, or subselective catheterizations. In these instances, the required pressures are greater, usually 20 to 40 psi. Although discouraged, the use of 4F or 5F systems should allow for constant low-pressure (10-20 psi) infusion throughout the administration. • Irrespective of the size of the catheter system, it is necessary to ensure that the rate of infusion mimics the rate of hepatic arterial flow. This rate is assessed by visual inspection of a test dose of contrast infusion prior to TheraSphere administration. In some patients (e.g., elderly with diminished cardiac function, celiac stenoses), slower hepatic arterial flow may be apparent. In such cases, the infusion rate of TheraSphere must mimic this slower flow. Injecting TheraSphere at an infusion rate faster than the vessel will tolerate can result in reflux and administration to nontarget sites. • Delivery of TheraSphere is dependent upon blood flow through the hepatic vasculature distal to the catheter tip. Therefore, it is necessary to make certain the catheter does not occlude the vessel in which it is placed, since doing so will result in vessel spasm and reflux as the infusion proceeds. • Most SIR-Spheres infusions should be performed through 3F catheter systems. Although smaller catheter systems result in the need to generate higher pressures for microsphere delivery, they create a safety mechanism preventing the forceful and unimpeded advancement of microspheres that might occur with larger 5F catheters. • There is no pressure gauge on the SIR-Spheres delivery kit, so pressure cannot be monitored. • Delivery of SIR-Spheres is dependent on blood flow through the hepatic vasculature distal to the catheter tip. Given the embolic load of SIR-Spheres, it is necessary to make certain the catheter does not occlude the vessel in which it is placed in order to prevent reflux. Flushing should be continued until optimal delivery is achieved. • It is essential that the vascular bed providing blood flow to the liver be altered in a manner comparable to that achieved using surgical techniques when placing surgical pumps. This includes prophylactic embolization of the GDA and right gastric arteries as well as the supraduodenal, falciform, accessory left gastric, and accessory inferior phrenic (extrahepatic). Once this is achieved, given the fact that metastatic cancer is usually multifocal and bilobar, treatment using a lobar approach is favored, thereby minimizing the risk of hepatic decompensation. • Given the large number of microspheres (40-80 million) and lower activity of SIR-Spheres (50 Bq per microsphere), the delivery of SIR-Spheres is distinctly different than that for TheraSphere. Fluoroscopic guidance is essential during the infusion. The technique of SIR-Spheres infusion involves the alternating infusion of sterile water and contrast, never allowing direct SIR-Spheres contact with contrast. This allows the authorized user to adequately monitor the injection and ensure that vascular saturation has not been reached. • The infusion is complete if either: (1) the entire intended dose has been infused without reaching stasis, or (2) stasis has been reached and only a portion of the dose has been infused. Given the risk of reflux and nontarget radiation once stasis has been reached, continued infusion of SIR-Spheres is not recommended.
Radioembolization of Liver Metastases
Clinical Relevance
Indications
Contraindications
ECOG Scale
Characteristics
Equivalent Karnofsky Score
0
Asymptomatic and fully active
100%
1
Symptomatic, fully ambulatory, restricted in physically strenuous activity
80%-90%
2
Symptomatic, ambulatory, capable of self-care, more than 50% of waking hours are spent out of bed
60%-70%
3
Symptomatic, limited self-care, spends more than 50% of time in bed
40%-50%
4
Completely disabled, no self-care, bedridden
20%-30%
Technique
Anatomy and Approaches
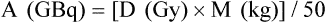
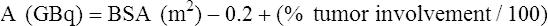
Technical Aspects
TheraSphere Infusion Technique
SIR-Sphere Infusion Technique
Radiology Key
Fastest Radiology Insight Engine