Introduction
The use of radiofrequency energy to create focal thermal tissue injury was first studied in the liver in the early 1990s. In 1996 the first radiofrequency ablation (RFA) procedure was performed on a patient with a liver tumor at Massachusetts General Hospital. RFA has since been used for various indications including unresectable tumors and cardiac arrhythmias. This technology has also been successfully applied to selective fetal reduction in cases of complex monochorionic gestations discordant for growth or congenital anomalies, twin-twin transfusion syndrome (TTTS), or twin reversed arterial perfusion (TRAP) sequence.
As a result of vascular anastomoses that link twin circulations within a shared placenta, cardiovascular disturbances affecting one twin in a monochorionic pair can present substantial risk of morbidity and mortality to the co-twin. On spontaneous demise of a monochorionic twin, transient hemodynamic changes occur that place the co-twin at risk for hypoxic-ischemic injury resulting in death (12%) or, if the fetus survives, profound neurologic injury (18%).
Because of the presence of a shared placental circulation, usual methods for selective fetal reduction in the setting of dichorionic twins are inappropriate in the setting of monochorionicity. In particular, intracardiac potassium chloride instillation, for selective termination, is contraindicated because this may trigger hemodynamic instability that affects the nontargeted twin. There is also the theoretic risk of injury to the nontargeted fetus as a result of bolus potassium chloride transfer between fetuses.
Techniques for selective fetal reduction within a monochorionic twin or higher-order multiple gestation are intended to achieve simultaneous occlusion of all umbilical vessels within the target fetus, minimizing the risk of periprocedural co-twin insult. An RFA-based, ultrasound (US)-guided percutaneous approach to achieve cord occlusion has become a popular therapeutic option because of its apparent safety and efficacy. The most robust published documentation of RFA involves its application for reduction of an acardiac twin with a TRAP presentation. By interrupting blood flow to the acardiac fetus, the “pump” co-twin is relieved of excess cardiovascular burden, preventing progression to high-output heart failure ( Chapter 164 ).
The literature documenting experience with RFA application for other discordant abnormalities with monochorionic twins is limited. Case reports reveal a range of procedural outcomes, but data regarding short-term and long-term outcomes or procedural risks are limited. In particular, data for long-term neurologic outcomes in survivors of technically successful RFA procedures are scarce.
Procedure
Description, Technique, and Equipment
RFA selective fetal reduction uses a minimally invasive, US-guided percutaneous technique for targeted umbilical cord occlusion. High-frequency sinusoidal current (400 to 500 kHz) creates thermal energy at a focused target tissue site. To disperse this energy, the generator is connected to the patient through grounding pads applied bilaterally to the anterior and posterior aspects of the thighs. Current is conducted through the patient to a specialized probe and then back to the generator, completing the circuit. RFA probes contain an insulated shaft and a noninsulated distal tip. Some probes contain deployable tines, which enable the user to secure the probe within a target tissue site and allow adjustment of the diameter for tissue ablation. The energy released at the exposed tip produces ionic agitation and frictional heat, which at the appropriate temperatures leads to cell death and coagulation necrosis.
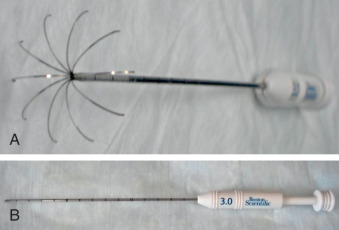
At Columbia University Medical Center, RFA for complicated monochorionic twin and higher-order multiple gestations is generally restricted to pregnancies between gestational age 16 weeks, 0 days and 23 weeks, 6 days; US evidence of amnion-chorion fusion is a further prerequisite. We perform most RFA procedures in an outpatient setting under local anesthesia after having the patient take oral opioid analgesia; however, selected cases with concern for extreme technical difficulty or patient pain intolerance are performed under regional anesthesia in an operating room. We use the RF 3000 Generator (Boston Scientific, Natick, MA; Fig. 114.1 ) and the LeVeen SuperSlim Needle Electrode RFA probe (Boston Scientific; Fig. 114.2 ). Table 114.1 lists all equipment needed for the procedure. The LeVeen SuperSlim Needle Electrode is preferred because of its narrow gauge and deployable tines. A needle without tines can also be used in certain circumstances, including cases with oligohydramnios where movement of the target site tends to be limited. However, displacement of needles without tines can occur with fetal movements in cases with normal or increased amniotic fluid.

|
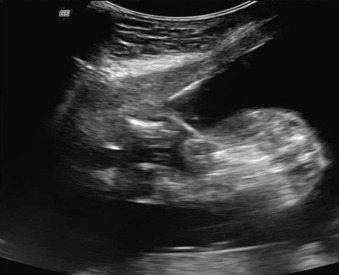
We routinely administer intravenous cefazolin as preoperative antibiotic prophylaxis, although not all centers use antibiotics. After placing the patient in the supine position with a slight leftward tilt, grounding pads are applied. After adequate anesthesia has been administered, US is used to locate both fetuses, the shared placental mass, and the dividing membrane. Discordant US findings are visualized, and designation of the appropriate target fetus is confirmed a second time. An intrafetal target site is identified near the umbilical cord insertion into the abdominal wall of the target fetus. A No. 11 blade scalpel is used to create a 5-mm skin incision on the maternal abdomen at the corresponding location along the intended entry approach. A needle electrode is advanced through this incision to the fetal target site under continuous US guidance, taking care to avoid traversing the dividing membrane ( Fig. 114.3 ). Whenever possible, a transplacental approach is avoided.
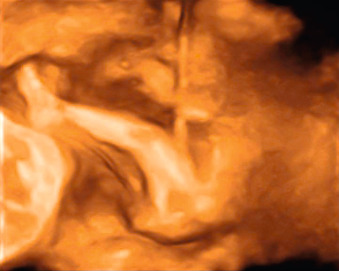
After confirmation of an appropriate target, using both two-dimensional and color Doppler imaging ( Fig. 114.4 ), the radial tines of the probe are deployed to a predetermined diameter ( Fig. 114.5 ). Energy is gradually increased until complete tissue impedance has been achieved, usually over a period of roughly 1 to 2 minutes. Doppler is then used to confirm cessation of blood flow within all target fetus umbilical vessels. Persistent fetal bradycardia within the targeted fetus usually indicates technical success, although cardiac activity can persist for hours within the targeted fetus after the umbilical vessels have been successfully coagulated. After a sustained absence of blood flow is confirmed within the target cord, the tines are fully retracted within the needle sheath, and the RFA probe is removed.