CHAPTER 25 Radiopharmaceuticals and Radiation Dose Considerations in Cardiac Positron Emission Tomography and PET/CT
PET RADIOPHARMACEUTICALS
Perfusion Agents
There are currently three PET perfusion agents that are approved for clinical use (13N-ammonia, 15O-water, and 82Rb), although several other PET perfusion agents are under preclinical and clinical evaluation. We focus on the clinically available agents (Table 25-1).
13N-Ammonia
13N-Ammonia is a cyclotron produced PET perfusion agent with a physical half-life of 10 minutes requiring an on-site cyclotron and radiochemistry synthesis capability. In the blood, 13N-ammonia exists in equilibrium between two states, neutral ammonia (NH3) and charged ammonium (NH4+). The neutral ammonia rapidly crosses cell membranes. Once it is inside the cell, glutamine synthase takes glutamate, ammonia, and ATP and produces glutamine, which remains intracellular, thereby fixing the 13N within the cell with very little diffusion back into the blood pool. 13N-Ammonia has a high extraction rate, with a significant percentage of the agent entering the myocyte.1 Animal models demonstrate, however, a nonlinear extraction of 13N-ammonia compared with the gold standard of microsphere flow measurements. At flows in the normal resting physiologic range of about 50 to 150 mL/min/100 g, 13N-ammonia uptake is nearly linear. However, at higher flows of more than 200 mL/min/100 g, such as those produced with vasodilator stress, there is a plateau effect, and higher flow rates are not associated with a further linear increase of 13N-ammonia uptake and retention.2 The nonlinear uptake at higher flow rates does not reflect a limitation of the diffusion of 13N-ammonia; rather, it is a limitation of metabolic trapping through the glutamine synthase pathway. This roll-off phenomenon may lead to underestimation of the true myocardial perfusion at higher flows, a statement true for all extracted radiotracers (Fig. 25-1). 13N-Ammonia image quality is generally excellent, with rapid clearance of the tracer from the blood pool and lung, although lung uptake can be an issue in patients with chronic lung disease or depressed left ventricular function.3
15O-Water
15O-Water is also a cyclotron-produced agent with an ultrashort half-life of 2 minutes. Unlike 13N-ammonia and 82Rb, 15O-water is a diffusible agent that is not extracted by or trapped within the myocytes but rather freely diffuses across membranes. The kinetics of 15O-water are dependent only on myocardial perfusion and are not affected by the metabolic rate-limiting steps that affect extracted tracers, eliminating the issues with tracer roll-off. However, PET 15O-water imaging generally is performed only as a dynamic study to determine uptake kinetics. Application of a single-compartment model to the dynamic image data allows a highly accurate estimation of absolute myocardial flow across a wide range of blood flow rates (0.29 to 5.04 mL/min/g).4 15O-water can be administered as an intravenous bolus, as a slow infusion, or by inhalation of 15O-CO2, which is converted to 15O-water in the lungs by carbonic anhydrase.
The advantage of 15O-water as an “ideal” flow tracer is counterbalanced by the complex acquisition protocols and analyses required for use in clinical practice. Because the 15O-water is not extracted from the blood pool, the images are contaminated by the high level of residual activity within the blood pool, which must be subtracted from the final image to properly evaluate myocardial perfusion. Two techniques are used to accomplish this goal. The blood pool can be labeled with 15O-carbon monoxide (CO). Inhalation of 15O-CO leads to irreversible binding of the CO to hemoglobin, thereby labeling the red blood cells and the blood pool. Blood pool data obtained during this acquisition can be subtracted from the 15O-water perfusion, eliminating the contribution of the blood pool from the final 15O-water data set. Alternatively, very early acquisitions (20 to 40 seconds after tracer infusion) provide an image of the blood pool before tracer flow has reached the myocardium; these are then used to remove background blood pool signal from the myocardium.5 The difficulties in image processing as well as the increased radiation exposure to the lung with use of the inhaled 15O-CO technique limit the clinical application of 15O-water.
Rubidium 82
82Rb is a radioactive monovalent cationic potassium analogue. Unlike the other perfusion agents used in cardiac PET, 82Rb is produced in a commercially available generator, alleviating the need for an on-site cyclotron, which makes it a more practical perfusion tracer for widespread clinical application. 82Rb is obtained by elution of 25 to 50 mL of normal saline with use of a computerized pump through a strontium 82 (82Sr) generator. The generator is rapidly replenished, with 90% of the maximal activity available after 5 minutes, and full replenishment occurs by 10 minutes. 82Rb has an ultrashort half-life of 75 seconds, which allows rapid acquisition of sequential stress and rest images (Fig. 25-2). The half-life of 82Sr is 25.5 days, and therefore the generator needs to be replaced every 4 weeks.
82Rb is extracted from the blood pool by the Na+,K+-ATPase pump. Extraction of 82Rb is similar to that of thallium 201 (201Tl), another potassium analogue,6 but it is less than that of 13N-ammonia7; therefore, the issues associated with roll-off may be present in 82Rb as they are in all other extractable tracers. Extraction of 82Rb is affected by myocardial perfusion as well as by the metabolic milieu. Severe acidosis and hypoxemia can affect 82Rb extraction.6 In addition, 82Rb is a less ideal imaging agent because the 82Rb positron has a high kinetic energy, which allows the positron to travel a relatively long distance before an annihilation event occurs. The increased positron range of the parent molecule results in a reduced spatial resolution of 82Rb imaging compared with that possible with other PET agents. Despite these issues, 82Rb has become one of the most commonly used PET perfusion agents because of the availability, ease of use, and acceptable image quality.
Summary of PET Perfusion Imaging
PET perfusion has become a routine tool in the management of cardiac patients. Multiple studies have evaluated the accuracy of perfusion PET for the detection of coronary artery disease. PET has consistently been found to have a high sensitivity and specificity for the detection of coronary artery disease. The accuracy of this technique makes the use of PET perfusion an attractive option in patients with chest pain or suspected coronary artery disease despite the increased cost and relative technical complexity (Table 25-2).8–15
Metabolic Agents
18F-Fluorodeoxyglucose (18F-FDG)
Fluorodeoxyglucose (2-fluoro-2-deoxy-d-glucose, FDG) is a glucose analogue that enters the cell through facilitated diffusion. Once inside cells, it is phosphorylated by hexokinase into FDG 6-phosphate, which is metabolically inert and is trapped within the myocyte. Therefore, retention of FDG is an energy-requiring process and is dependent on intact metabolic pathways (Fig. 25-3). FDG can be labeled with fluorine18 (18F), which decays by positron emission with a half-life of 110 minutes. The prolonged half-life of 18F allows local distribution of the agent from a central cyclotron site to regional imaging centers. Whereas it is the most commonly used agent in oncologic PET imaging, 18F-FDG is used primarily for the evaluation of myocardial viability in cardiac imaging.
Preparation of the patient is important in the proper acquisition of 18F-FDG images. In the fasting state, glucose uptake is low and inhomogeneous. To maximize glucose use by the myocardium, the patient is asked to fast for 6 to 12 hours. An oral glucose load (25 to 100 g) or an intravenous glucose load is administered and followed by insulin as needed before imaging. In diabetic patients, a more rigorous methodology is necessary to produce high-quality scans, and a euglycemic hyperinsulinemic clamp is often used. A dose of 5 to 15 mCi is injected, and imaging begins at least 45 minutes after tracer infusion. Viability studies with 18F-FDG are performed in conjunction with a perfusion study. Perfusion can be acquired with PET perfusion agents as described before or with SPECT perfusion agents such as 201Tl or technetium Tc 99m. Preserved FDG uptake in the setting of reduced perfusion suggests the presence of viable myocardium. These segments are likely to have improved function after revascularization. Segments that have both diminished perfusion and diminished FDG uptake are unlikely to recover function with revascularization and consist mostly of infarcted tissue (Fig. 25-4).
11C-Palmitate and 11C-Acetate
As noted previously, free fatty acids are the primary source of energy in normal myocardium, accounting for 60% to 80% of ATP produced in nonischemic myocytes. Free fatty acids are activated as acyl coenzyme A. Acyl coenzyme A enters the mitochondria through the acyl carnitine transport system and becomes part of the beta-oxidation pathway. Whereas this pathway is a rich source of ATP, it is highly oxygen dependent. During periods of ischemia, there is a rapid shift away from fatty acid metabolism to increased glucose use (see Fig. 25-3). Free fatty acids account for the preponderance of myocardial energy formation, and these pathways are dramatically altered by ischemia. Therefore, free fatty acid imaging is an attractive target for noninvasive imaging of ischemia and the associated alterations in oxidative metabolism. PET imaging of fatty acid metabolism generally involves imaging with carbon 11 (11C)–labeled medium-sized straight-chain fatty acids like palmitate, which undergo beta-oxidation. 11C has also been used to label acetate as an alternative approach for imaging of oxidative metabolism. 11C is cyclotron produced and has a relatively short half-life of 20.4 minutes. Initially produced in 1934 and first studied in humans in 1945, it has the advantage of being an organic molecule and therefore can potentially be used to target a wide variety of metabolic processes.16 Unfortunately, 11C-labeled metabolic agents are more difficult to employ in clinical practice and have been primarily used for research purposes. However, both 11C-palmitate and 11C-acetate have been effectively employed for the evaluation of myocardial oxidative metabolism.
Several studies have evaluated fatty acid metabolism in normal volunteers and patients. Walsh and colleagues17 demonstrated decreased mitochondrial metabolism in infarcted myocardium. In addition, 11C-palmitate infusion during dobutamine stress testing demonstrated the expected rise in fatty acid metabolism in the normal myocardial segments, whereas areas supplied by stenosed vessels did not show a rise in fatty acid use.18 Last, free fatty acid metabolism imaging has been used to demonstrate differences in myocardial metabolism in the elderly19 as well as in inherited nonischemic cardiomyopathies.20 In both cases, fatty acid metabolism was decreased compared with normal controls. Although the clinical application of some of these findings is not fully elucidated, free fatty acid PET offers a powerful method for the in vivo evaluation of myocardial metabolism and therefore provides an important tool for gaining insight into disease processes associated with altered myocardial metabolism.

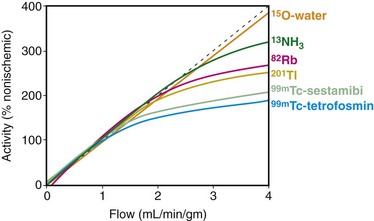
 FIGURE 25-1
FIGURE 25-1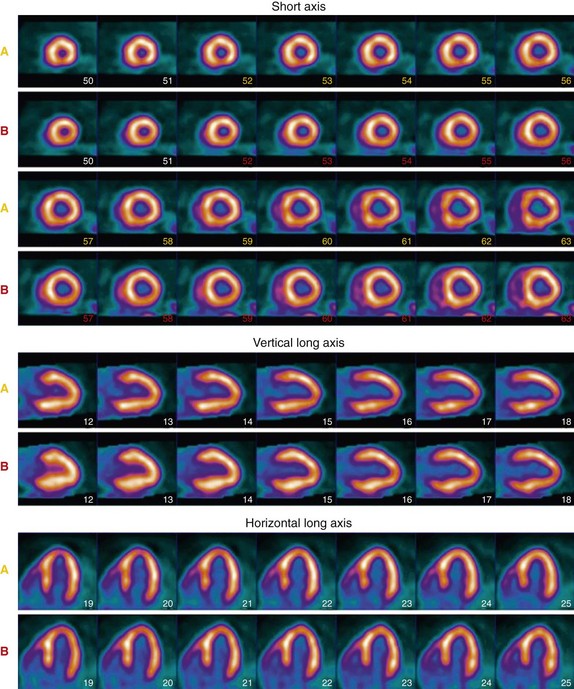
 FIGURE 25-2
FIGURE 25-2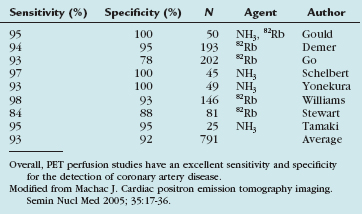
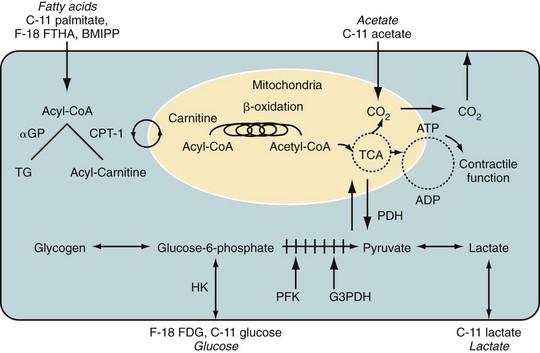
 FIGURE 25-3
FIGURE 25-3
 FIGURE 25-4
FIGURE 25-4


