CHAPTER 107 Renal Arteries
Computed Tomographic and Magnetic Resonance Angiography
INTRODUCTION
Renal computed tomographic angiography (CTA) and magnetic resonance angiography (MRA) have become clinically important diagnostic tools. Technological advancements are primarily responsible for their rapid development. The implementation of multidetector row array and slip-ring gantry design have propelled CT technology.1 In the past few years, there has been tremendous progress in the development of MR hardware and sequence design, high-performance gradients, parallel imaging techniques, and advanced torso phased-array coils enabling faster scanning and improved image quality. In contrast to CT, MR scanning does not utilize ionizing radiation, avoids exposure to nephrotoxic iodinated contrast material, and demonstrates the capability to provide functional information.2 Alternatively, CT scanning is more widely available and less expensive. Ultimately, although digital subtraction angiography (DSA) remains the gold standard for diagnosing renovascular disease, the diagnostic accuracy of renal CTA and MRA and their ability to offer a reliable, less invasive alternative to diagnostic DSA have fueled their widespread adoption. In this chapter, the technical considerations for performing a renal CTA and MRA will be reviewed.
DESCRIPTION OF TECHNICAL REQUIREMENTS
Renal CTA
Renal CTA is best performed with a multidetector row CT (MDCT) scanner, especially one with at least 64 detector rows. A dual-chamber power injector and a dedicated 3D workstation are also recommended. On-going CT hardware and software development have focused on optimizing image quality while minimizing radiation exposure and improving coordination of the scan with contrast material administration.1
TECHNIQUES
Indications
The most common indications for renal artery CTA and MRA include evaluation for renal artery stenosis and evaluation of potential living kidney donors.1–3 Other common indications include presurgical planning for ureteropelvic junction (UPJ) obstruction, renal tumor evaluation, assessment of congenital anomalies, and trauma.1,3
Renal Artery Stenosis
Renal artery stenosis most commonly occurs secondary to atherosclerosis.4,5 Atherosclerotic disease generally occurs in older patients. Often, there is generalized or diffuse atherosclerotic plaque formation with concomitant involvement of the aorta and the ostium or proximal segment of the main renal artery (Fig. 107-1).4,5 The second most common etiology is fibromuscular dysplasia (FMD) which is found in younger patients, typically females.2,4,5 As opposed to atherosclerotic disease, the middle and distal segments of the main renal artery tend to be more affected in FMD (Fig. 107-2).2,4,5
The clinical presentation of patients with renal artery stenosis may include abrupt onset or intractable hypertension, acute or chronic azotemia, angiotensin converting enzyme inhibitor induced azotemia, asymmetric renal size, and congestive heart failure in patients with normal ventricular function.2 CTA demonstrates a sensitivity up to 90% and a specificity up to 97% in the detection of hemodynamically significant renal artery stenosis.6 MRA demonstrates a sensitivity of 88% to 100% and a specificity of 70% to 100% in the diagnosis of renal artery stenosis, especially for severe stenosis greater than 70%.2 Following therapeutic angioplasty and/or stenting for renal artery stenosis, CTA and MRA may also be helpful for the surveillance of renal artery restenosis.
Renal Donor Evaluation
Many renal transplantations are performed with living renal donors because of the insufficient availability of cadaveric kidneys.7 Accurate evaluation of the renal vasculature, parenchyma, and collecting system is critical for determining suitability of the donor kidney for transplantation and for preoperative planning.7–10 In many medical centers, donor nephrectomy is now performed laparoscopically to diminish morbidity for the donor.7–10 Consequently, accurate preoperative evaluation has become even more important to minimize the likelihood for unexpected intraoperative vascular complications from unexpected anatomic variants. More than one third of patients may possess anatomic variants (Fig. 107-3).5,7–10 This statistic highlights the importance of accurate preoperative assessment.
Preoperative Planning
In preoperative planning for congenital UPJ obstruction surgery, the CTA or MRA is one component of the diagnostic imaging evaluation. The renal and vascular anatomy, etiology of obstruction, functional significance of obstruction and concomitant conditions will determine the treatment approach.11 The CTA or MRA can typically identify a crossing vessel that is responsible for extrinsic compression of the UPJ resulting in obstruction.11 CTA demonstrates a sensitivity of 91% to 100% and a specificity of 96% to 100% in the detection of crossing vessels.11 MRA demonstrates an accuracy of 86% in the detection of a crossing vessel.11 The presence of a crossing vessel may necessitate a more invasive surgical approach to treat the UPJ obstruction.11
Renal Tumor Evaluation
Renal tumor evaluation is another indication for renal artery CTA and MRA. The vascular features of the tumor may assist staging of the tumor and presurgical planning.11 For instance, renal cell carcinoma may invade the renal vein and extend into the inferior vena cava, which necessitates a substantially longer and more extended surgical approach. Renal cell carcinoma extension into the venous system is typically well assessed by CTA and MRA. Also, the detection of accessory renal arteries or possibly extracapsular feeding arteries of a renal malignancy are important preoperative determinations because knowledge of their presence will minimize intraoperative bleeding complications, especially for laparoscopic nephrectomies, which are becoming increasingly popular surgeries.11
Contraindications
Renal CTA
The contraindications for renal CTA include impaired renal function, known allergy to iodinated contrast material and pregnancy. Also, caution is advised in diabetic patients taking metformin hydrochloride. The development of acute renal impairment secondary to contrast-induced nephropathy may prohibit proper clearance of metformin, which is eliminated through the kidneys, and result in lactic acidosis.12 These patients should discontinue use of metformin for at least 48 hours following administration of iodinated contrast material.12 Metformin may be resumed after 48 hours if the patient’s renal function is normal.12
Renal MRA
Recently, a rare and potentially life-threatening condition called nephrogenic systemic fibrosis (NSF) has been reported in patients with advanced renal failure receiving gadolinium-based contrast material.13,14 This disease is characterized by fibrosis of the skin and connective tissues throughout the body. Patients may develop skin thickening to such an extent that it results in decreased joint mobility. Fibrosis may also occur in other parts of the body including the diaphragm, musculature of the lower abdomen and extremities, and the pulmonary vasculature. The clinical course of NSF is progressive and may be fatal. At the present time, no treatment has been identified. Consequently, extreme caution is advised in administering gadolinium-based contrast material in those patients with advanced renal failure. At our institution, we do not recommend the use of gadolinium-chelate contrast agents in patients with glomerular filtration rate (GFR) < 30 mL/min/1.73 m2.
Technique Description
Renal CTA
The New York University renal CTA protocol is outlined in Table 107-1. Two important factors in performing a renal CTA examination are the selection of scan parameters for optimization of sufficient volume of anatomic coverage for the highest spatial resolution possible within a reasonable scan period, and the administration of iodinated contrast medium for sufficient opacification of the aorta and renal arteries during imaging. Multidetector row array technology and slip-ring gantry design allow volumetric coverage from the upper abdominal aorta through the common iliac arteries within one breath-hold. The data set may be reconstructed at different slice thicknesses to review the images. For a renal CTA, the data set are reconstructed with overlapping thin slices to enable improved data postprocessing on a 3D workstation.
TABLE 107-1 New York University Renal CTA Protocol
| Scan Parameters for a 64-slice MDCT Scanner | |
| Range | Top of kidneys through iliac crests |
| Collimation | 64 × 0.6 |
| Slice thickness | 1 mm |
| Increment | 0.5 mm |
| Pitch | 0.9 |
| Rotation time | 0.5 sec |
| kVp | 120 (Caredose) |
| mAs | 280 |
| CTDI vol | 20.2 mGy |
| IV contrast dose | 1.5 mL/kg (minimum 100 mL) |
| IV contrast infusion | 3-4 mL/sec |
| Scan delay | Bolus tracking at the level of T12 (threshold of 150 HU) |
CTDI vol, CT dose index of scanned volume.
The most critical element of performing a renal CTA examination is coordination of the scan delay with the arterial arrival of the intravenous contrast bolus.1 Ideally, the scan is obtained during peak contrast opacification of the aorta and renal arteries (Fig. 107-4). The contrast infusion duration should approximate the scan acquisition time.1 Faster scans require less contrast material. However, the drawback of using less contrast material is the shortened duration of the contrast bolus, which increases the challenge for proper coordination of imaging with opacification of the arteries. That is to say, if scanning is too fast, imaging may occur prior to complete opacification of the target vasculature, resulting in suboptimal arterial contrast enhancement and poor arterial visualization. Alternatively, if scanning is slow or performed too late, images would illustrate the venous phase of the bolus with suboptimal arterial enhancement, Consequently, it is important to accurately determine circulation time to achieve an optimal scan.
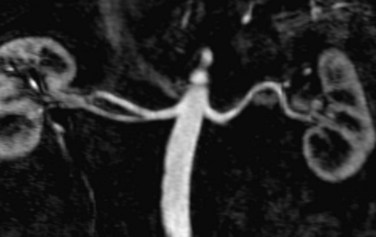
 FIGURE 107-4 Multiplanar reformation (MPR) image from a renal MRA demonstrating bilateral renal arteries.
FIGURE 107-4 Multiplanar reformation (MPR) image from a renal MRA demonstrating bilateral renal arteries.
There are three methods to determine the patient’s circulation time for proper scan delay determination.1
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


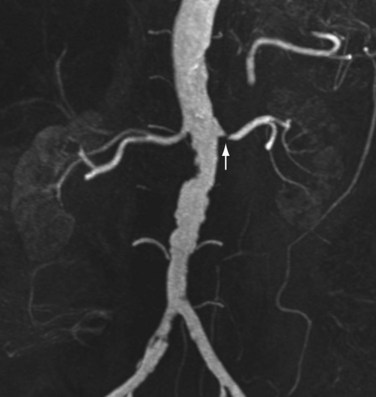
 FIGURE 107-1
FIGURE 107-1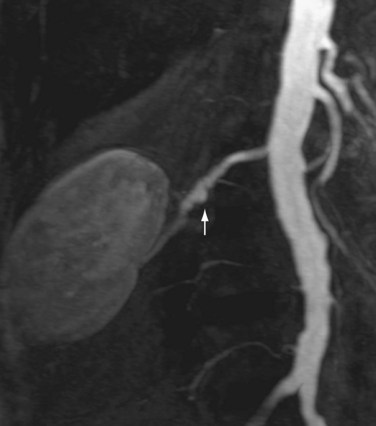
 FIGURE 107-2
FIGURE 107-2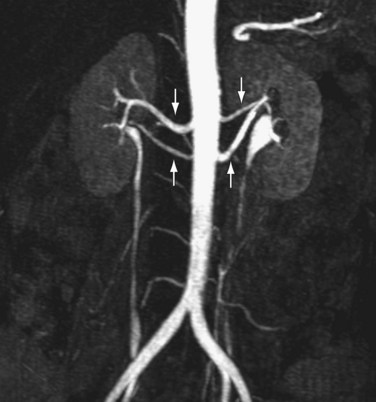
 FIGURE 107-3
FIGURE 107-3