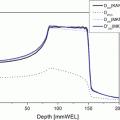
Fig. 28.1
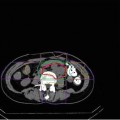
Dose distribution of the carbon ion therapy
The clinical target volume (CTV) should include a margin of 0–5 mm around the macroscopic tumor (gross target volume: GTV), ITV (internal margin) should include a margin of 5–10 mm (depends on the accuracy of respiratory gating) in the craniocaudal direction around the CTV, and the planning target volume should include 5–10 mm around ITV. The whole kidney is not required to be included in the irradiation field. An optimum ridge filter is used for adjustment of the spread-out Bragg peak (SOBP), a resinous bolus is manufactured for adjustment of depth and shape of irradiation field, and a multi-leaf collimator or metallic collimator is used for shaping the irradiation field. Irradiation is delivered once per day and 4 times per week (Tuesday to Friday) at the present institution.
Due to respiratory organ motion, respiration-synchronized irradiation using a respiration sensing system is essential for treatment. Because the location of the kidney (or tumor) is hard to identify with X-ray imaging, the implantation of one or more metallic markers (1–3 mm in length) under local anesthesia in the ipsilateral renal cortex around the tumor as target markers is required. Metallic markers should not be implanted in the tumor so that there is no influence on dose distribution and also no induction of tumor seeding. Two or three markers are desirable due to the risk of migration of the markers after implantation. All irradiations are performed identifying these permanent markers by X-ray radiography.
A total dose of 64–80 GyE/16 fr was used in past treatments, and favorable outcomes were obtained. Local recurrence occurred in one patient treated with 72 GyE/16 fr; however, it is not clear whether 72 GyE/16 fr is sufficient because of the marginal recurrence. A total dose of 66 GyE in 12 fractions is used in a phase II clinical trial in the present institution.
28.4.2 Organs at Risk
28.4.2.1 Gastrointestinal Tract
The most important organ at risk during RT for renal tumors is the gastrointestinal tract. It is difficult to treat when the ascending or descending colon is in contact with renal tumor. When it comes to a tumor located in the superior and anterior region of the kidney, attention to the duodenum, small intestine, and stomach should be required.
28.4.2.2 Liver
A part of the liver will be irradiated in case the tumor is in the cranial region of the right kidney. But there is less possibility to cause severe toxicity other than transient mild liver dysfunction.
28.4.2.3 Kidney/Renal Pelvis
It is possible to avoid renal function loss by preserving the ipsilateral normal kidney. Because atrophy of the renal pelvis leads to dysfunction of the upstream renal cortex, renal pelvis must be preserved as much as possible.
28.4.2.4 Adrenal Gland
Because it is on the bilateral side, there is less possibility of symptomatic adrenal insufficiency by ipsilateral adrenal dysfunction. Prior explanation would be essential when a patient has existing adrenal dysfunction.
28.4.2.5 Skin/Muscle
Body surface dose should be limited as follows: 60 GyE ≤ 20 cm2 to avoid ≥grade 3 late skin toxicity [35]. Muscle is less likely to be affected by radiation, but high-dose irradiation sometimes causes long-lasting dull pain.
28.4.2.6 Spinal Cord
It usually is not in close proximity to a renal tumor; however, in case the spinal cord is in contact with a tumor (T4 or massive tumor), high-dose irradiation to the spinal cord should be avoided.
28.5 Results of Therapy
28.5.1 Outcome of Carbon Ion Therapy
We previously reported the initial use of carbon ion therapy for primary RCC [36]. Patient characteristics are listed in Table 28.1. Nine patients had histologically proven RCC, including eight patients with clear cell carcinoma and one patient with mixed type; in addition, RCC was diagnosed in one patient without a biopsy. There were seven patients with clinical stage I (T1a N0: 5, T1b N0: 2) and three patients with stage IV (T4 N0: 2, T3a N2: 1) carcinoma. The median maximum diameter of the tumor was 4.3 cm (range, 2.4–12 cm), and the median initial tumor volume was 38.4 cc (range, 5.8–508.7 cc). The median follow-up for surviving patients was 86.2 months (8.1–137.8). Local recurrence was defined as definite regrowth of the tumor except for transient enlargement of the tumor after irradiation.
Table 28.1
Patient characteristics
No. | Age | Gender | Histology | TNM (UICC) | Total dose/fraction (GyE/16 fr) | Max diameter (cm) | Tumor volume (cc) | Local failure | Distant metastasis | Late toxicity | Follow-up period (months) | Dead or alive |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
1 | 67 | Male | RCC | T1bN0M0 | 72 | 5 | 52.33 | (–) | (–) | (–) | 118 | DID |
2 | 70 | Male | RCC | T1aN0M0 | 72 | 3.2 | 11.32 | (–) | (–) | (–) | 42.4 | DID |
3 | 59 | Male | RCC | T4N0M0 | 80 | 6.5 | 120.21 | (–) | (–) | (+) | 72.3 | NED |
4 | 55 | Male | RCC | T1aN0M0 | 80 | 2.4 | 5.8 | (–) | (–) | (–) | 137.8 | NED |
5 | 71 | Male | RCC | T1aN0M0 | 80 | 3 | 6.28 | (–) | (–) | (–) | 126.8 | NED |
6 | 69 | Male | RCC | T4N0M0 | 72 | 12 | 508.68 | (–) | (+) | (–) | 74.4 | DOD |
7 | 82 | Male | RCC | T1aN0M0 | 72 | 3 | 12.31 | (–) | (–) | (–) | 8.1 | DID |
8 | 55 | Male | – | T1aN0M0 | 72 | 3.6 | 24.42 | (–) | (–) | (–) | 89.5 | NED |
9 | 61 | Male | RCC | T1bN0M0 | 72 | 5 | 62.8 | (+) | (+) | (–) | 82.9 | AWD |
10 | 52 | Female | RCC | T3aN2M0 | 64 | 6.5 | 137.2 | (–) | (+) | (–) | 75.7 | AWD |
The 5-year local control rate, cause-specific survival, and overall survival were 89, 100, and 80 %, respectively (Fig. 28.2




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree