Fig. 1
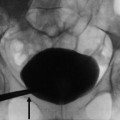
Chronic pyelonephritis. (a) Typical calyceal deformations. (b, c) Intravenous excretory urography. Progressive calyceal distortion (arrows) due to the underlying renal parenchymal damage and scarring
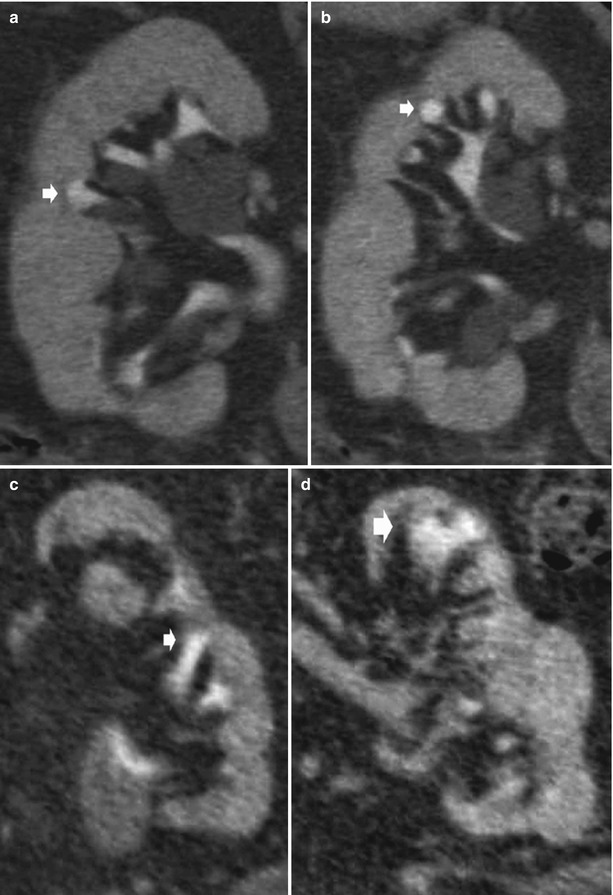
Fig. 2
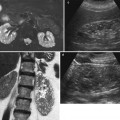
Chronic pyelonephritis. Multidetector CT urography. Coronal reformations. (a, b) Right kidney. Calyceal distortion and clubbing (arrows) due to the underlying renal parenchymal damage with renal parenchymal scarring and focal reduction of renal parenchymal thickness. (c, d) Left kidney. Diffuse reduction of the renal parenchymal thickness with calyceal distortion (arrows)
The goals of imaging in chronic recurrent pyelonephritis are the detection of chronic renal damage and the detection of abnormalities that are often the cause of recurrence. However it should be stressed that there are no radiological features that at a single point in time reliably indicate activity of the process. Therefore, the goals of radiological investigations become the detection of abnormalities that are often the cause of the recurrence (i.e., infectious stones) and the detection of the chronic renal damage. In the past, intravenous excretory urography and nephrotomography and, nowadays, CT urography are often capable to achieve these two goals. Findings related to the chronic renal damage (decrease of the kidney size, parenchymal scars, calyceal distortion; Fig. 1) are easily depicted at intravenous excretory urography.
Multidetector computed tomography (CT) urography and magnetic resonance (MR) imaging readily identify the chronic renal parenchymal damage and calyceal distortion and clubbing (Fig. 2).
2 Renal Tuberculosis
Genitourinary tuberculosis is the most common manifestation of extrapulmonary tuberculosis (Engin et al. 2000; Harisinghani et al. 2000) accounting for 15–20 % of infections outside the lungs (Gibson et al. 2004). Approximately 4–8 % of patients with pulmonary tuberculosis will develop clinically significant genitourinary infection (Gibson et al. 2004). Mycobacterium tuberculosis reaches the genitourinary organs, particularly the kidneys, by the hematogenous seeding from disease in the lungs. The seeding occurs at the time of the initial lung infection with seeding of Mycobacterium tuberculosis in the periglomerular and peritubular capillary bed. Small granulomas form in the renal cortex bilaterally, adjacent to the glomeruli, and remain stable for many years (Kenney 1990). A high rate of perfusion and favorable oxygen tension increase the likelihood of bacilli proliferating in this location (Gibson et al. 2004). In patients with intact cellular immunity, the disease remains confined to the renal cortex, while in some patients, breakdown of host defense mechanisms leads to reactivation of the cortical granulomas with enlargement and coalescence and organisms spread into the real medulla causing a papillitis which may extend into the collecting system (Kenney 1990). In fact, after capillary rupture the organisms migrate to the proximal tubule and loop of Henle with eventual development of enlarging, caseating granulomas and papillary necrosis. Granuloma formation, caseous necrosis, and cavitation are stages of progressive infection, which can eventually determine the loss of renal function and calcification of the entire kidney (autonephrectomy).
The renal disease remains quiescent until there is an insult to the host’s immunity at which time reactivation occurs. Patients with genitourinary tuberculosis typically have local symptoms including frequent voiding and dysuria. Hematuria can be either microscopic or macroscopic. Symptoms may also include back, flank, or abdominal pain (Simon et al. 1977; Gibson et al. 2004). Constitutional symptoms such as fever, weight loss, fatigue, and anorexia are less common (Simon et al. 1977; Gibson et al. 2004). Laboratory abnormalities include pyuria, proteinuria, and hematuria. Standard urine cultures can be normal. Furthermore, the presence of routine urinary tract pathogens can delay the diagnosis of coexistent tuberculosis (Gibson et al. 2004). Mycobacterium tuberculosis is isolated from the urine in 80–95 % of patients with genitourinary tuberculosis. In adults renal tuberculosis is the most known etiology of infundibular strictures with consequent hydrocalyx. Obstruction may develop early or during the healing phase, even while the patient is receiving antitubercular therapy.
It must be underlined that each finding of renal tuberculosis can be caused by other diseases, but multiple abnormalities are usually present and allow a correct diagnosis. That’s why renal tuberculosis is called the “great imitator.” On the plain film and unenhanced CT, the kidney may appear large, normal sized, or small. Despite hematogenous seeding of both kidneys, clinically significant disease is usually limited to one side and approximately 75 % of renal tuberculous involvement is unilateral.
The grayscale US appearance of renal tuberculosis is not specific. US is advisable to evaluate the nonfunctioning kidney after iodinated contrast agent injection and for the follow-up of antitubercular therapy. The kidney may appear large, normal sized, or small, and calcifications are common. Hydronephrosis or hypoechoic parenchymal lesions, which correspond to parenchymal abscesses resulting from caseating necrosis, may be observed.
CT urography, as in the past intravenous excretory urography, is now considered the correct imaging technique to assess the upper urinary tract, including the involvement in renal tuberculosis. The most common CT finding is renal calcifications (37–71 %) (Gibson et al. 2004) which follow different patterns. Calcifications may be amorphous, granular, lobar, or curvilinear and frequently extend beyond the kidney (e.g., psoas muscle).
CT urography allows a more accurate evaluation of the amount of residual functioning parenchyma and of the extrarenal spread (Kenney 1990). Nowadays CT urography represents the most accurate imaging technique to reveal early manifestations of renal tuberculosis (Fig. 3). The earliest morphologic alterations of renal parenchyma corresponding to calyceal alterations determined by tuberculosis include calyceal erosion (“moth-eaten calyx”) (Fig. 4a) with progression toward medullary (Fig. 4b) or papillary necrosis (Fig. 4c, d). The pathological features of renal tuberculosis are extremely different, and frequently different features coexist (Fig. 4b). Common sites of tuberculous strictures are the calyceal neck with hydrocalyx (Figs. 5a and 6) or phantom calyx (Figs. 5b–d and 7); the infundibulum of a calyx with hydrocalyx or regional or focal hydrocalycosis (Fig. 8); the ureteropelvic junction with dilatation of the entire renal pelvis, calyces, and infundibula (Fig. 9); and the lower ureteral segment. Tubercular strictures often coexist with adjacent renal parenchymal scarring. The development of infundibular, pelvic, or ureteral strictures is nearly pathognomonic of renal tuberculosis (Kenney 1990). An infundibular stricture may result in a “phantom calyx” when that segment of the kidney becomes nonfunctional (Fig. 5b–d) (Kenney 1990). CT is very accurate in demonstrating parenchymal gross calcifications (Fig. 8).
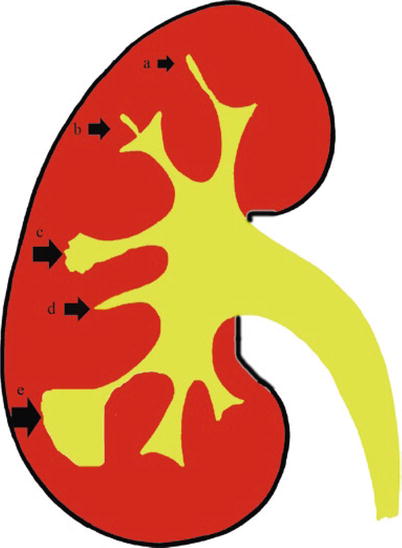
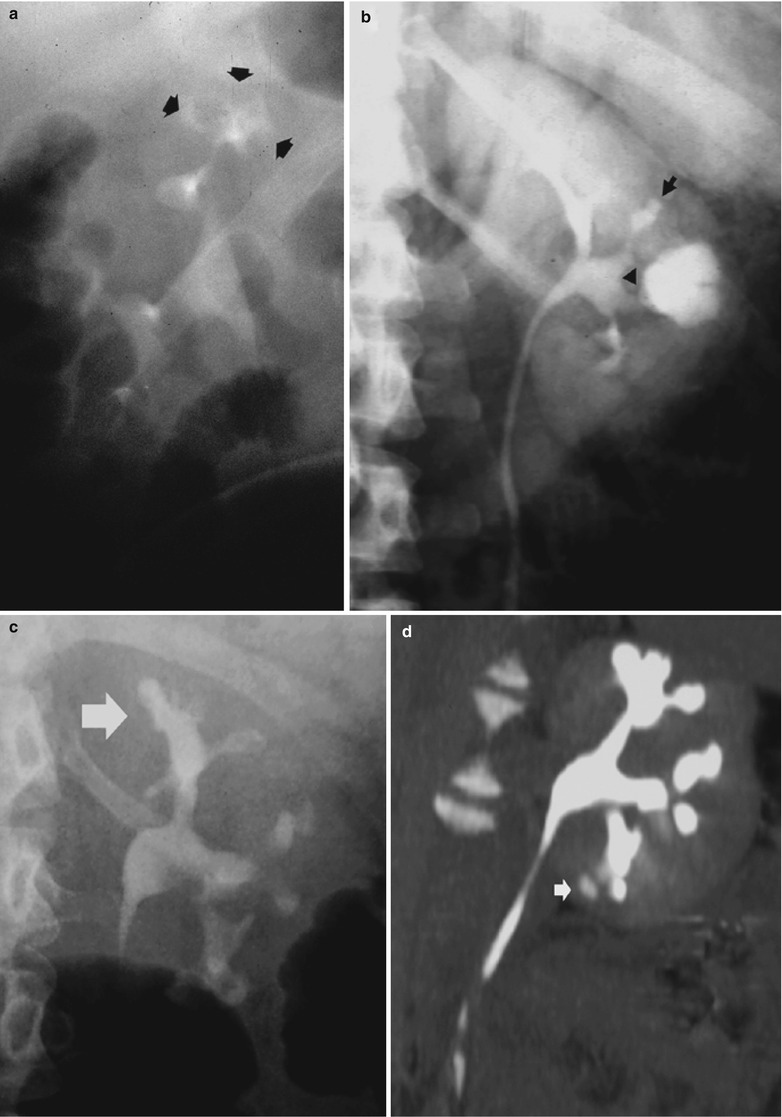
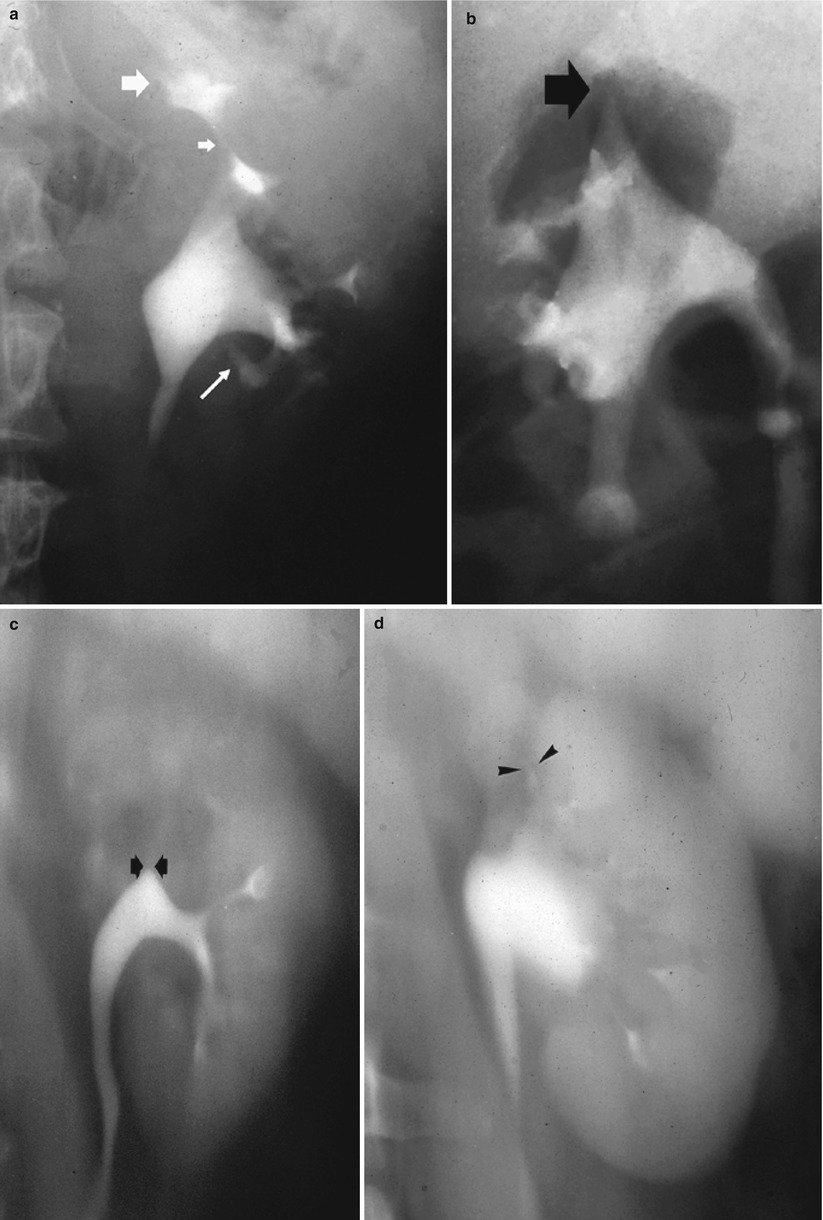
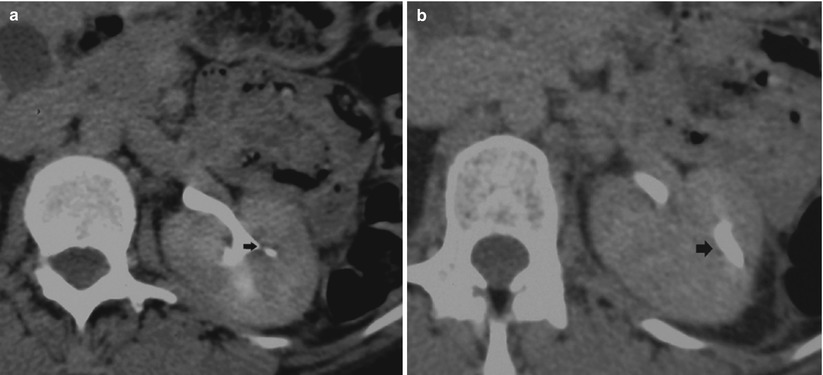
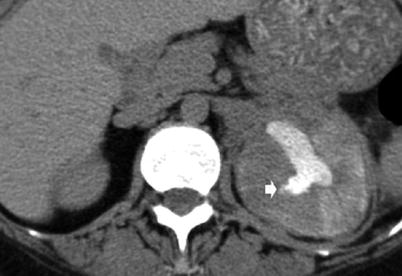
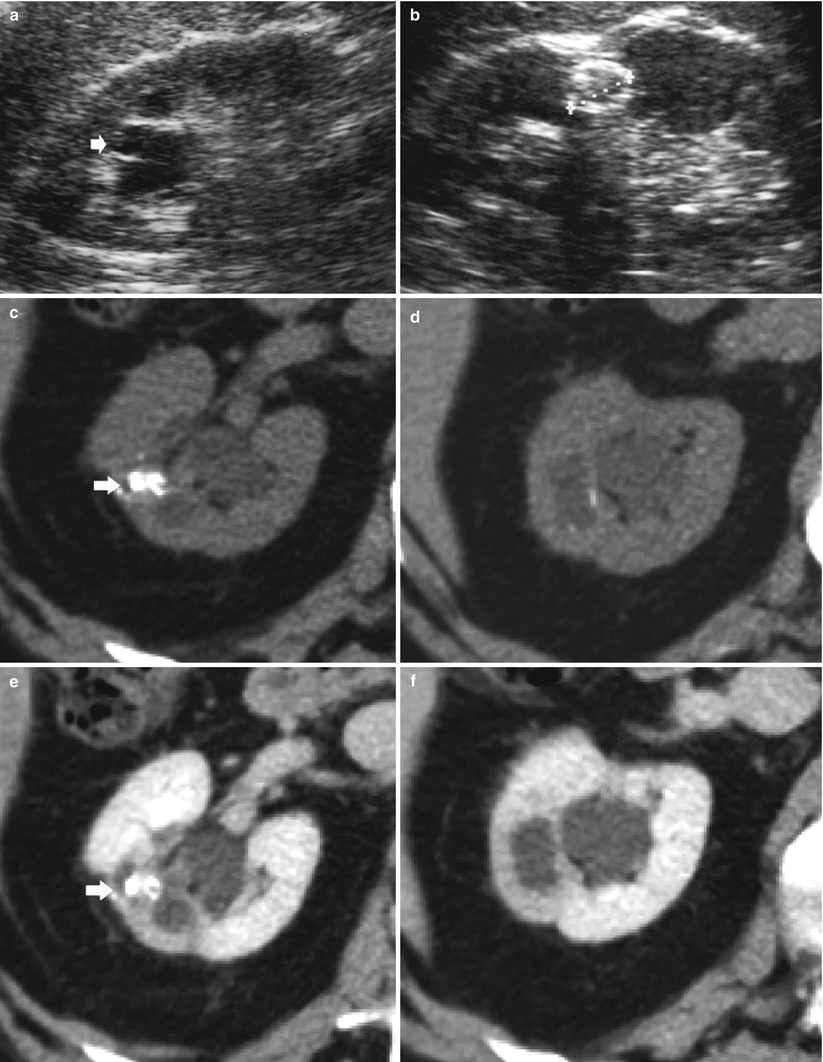
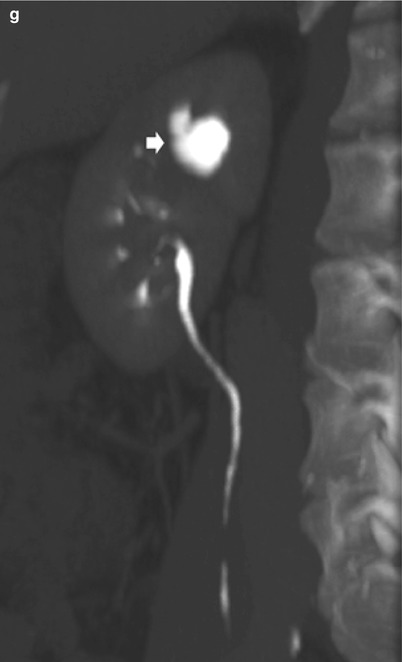
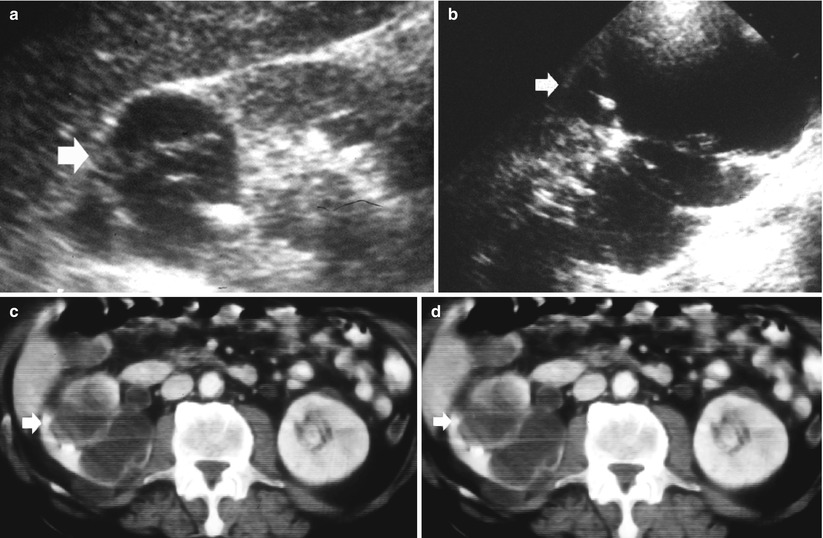

Fig. 3
The early morphologic alterations of renal parenchyma determined by tuberculosis. The fundamental calyceal alterations in renal tuberculosis: calyceal erosion (a), medullary necrosis (b), papillary necrosis (c), and infundibular stricture without (d) or with hydrocalyx (e)

Fig. 4
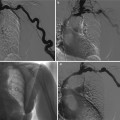
Renal tuberculosis. (a) Intravenous excretory urography. Calyceal distortion due to initial erosion (small arrows). (b) Intravenous excretory urography. Renal medullary necrosis (arrow) with evidence also of infundibular stricture (arrowhead) and adjacent hydrocalyx. (c) Intravenous excretory urography. Papillary necrosis (arrows). (d) CT urography. Maximum intensity projection. Renal medullary necrosis (arrow) in one of the renal calyces of the lower group

Fig. 5
Renal tuberculosis. (a) Intravenous excretory urography. Tubercular stricture (small arrow) with evidence of hydrocalyx (large arrow) and adjacent calyceal erosion (long arrow). (b) Intravenous excretory urography and (c, d) nephrotomography. Tubercular infundibular stricture (arrows) with phantom calyx. (d) Renal infundibular stricture (arrowheads) often coexists with adjacent renal parenchymal scar

Fig. 6
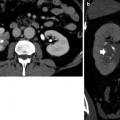
Renal tuberculosis. (a, b) CT urography. Transverse scan. (a) Infundibular stricture (arrow) due to fibrosclerosing tuberculosis with narrowing of the infundibulum. (b) Dilatation of an upper renal calyx (hydrocalyx)

Fig. 7
Renal tuberculosis. CT urography. Transverse scan. Infundibular stricture (arrow) due to fibrosclerosing tuberculosis with narrowing of the infundibulum and phantom calyx


Fig. 8
Renal tuberculosis. (a, b) Ultrasound scan, longitudinal view. Segmental dilatation of the upper urinary tract (arrow) with focal calcification within the renal parenchyma (calipers) and retraction of the renal profile. Unenhanced (c, d) and contrast-enhanced CT (e, f) show the renal parenchymal calcifications (arrow) with segmental dilatation of the intrarenal urinary tract. (g) CT urography, maximum intensity projection. Segmental dilatation of the upper urinary tract with hydrocalyx (arrow) due to fibrosclerosing tuberculosis with tuberculous stricture at the superior infundibulum with regional hydrocalycosis (arrow)

Fig. 9
A 63-year-old patient with renal tuberculosis. Contrast-enhanced nephrographic phase CT. US (a) transverse scan; (b) longitudinal scan. Dilatation of the renal pelvis (arrow), which is confirmed by contrast-enhanced CT (c, d). Renal tuberculosis determines a fibrosclerosis of the ureteropelvic junction with consequent markedly dilated calyces and renal parenchymal thinning
Renal tuberculosis may manifest as extensive cavitation (open or extensive forms) or fibrosclerosis (closed forms) (Becker 1988; Wang et al. 1997). The open or extensive form (Fig. 10) corresponds to noncommunicating parenchymal cavities or to communicating cavities with extension of the caseified tissue necrosis to the intrarenal excretory tract (Fig. 11). Parenchymal masses can develop which may be calcified (Kenney 1990). Communication of the granulomas with the collecting system (Fig. 12) can lead to regional spread of the bacilli into the renal pelvis, ureters, urinary bladder, and accessory genital organs. Extensive cavitation may determine renal caseation, whereas fibrosing reaction of the urinary tract results in obstructive hydronephrosis. When the process spreads into the collecting system, the disease can evolve in three ways: (1) extensive cavitation (Figs. 13 and 14), (2) fibrosclerosis with resulting noncommunicating cavities (Figs. 14, 15, 16 and 17), and (3) recurrent “poussées.”
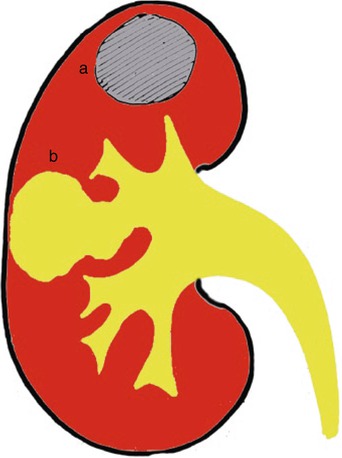
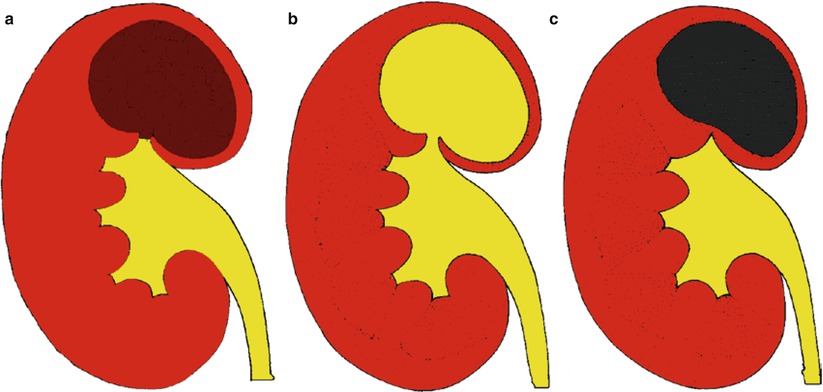
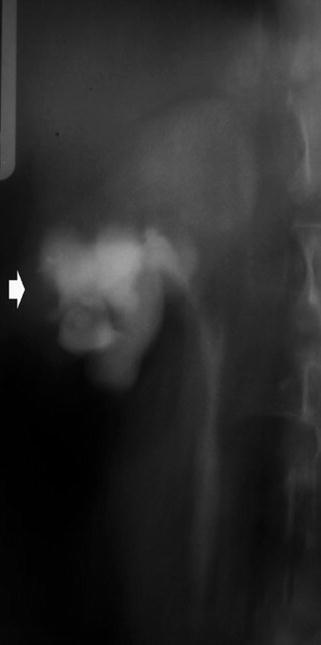
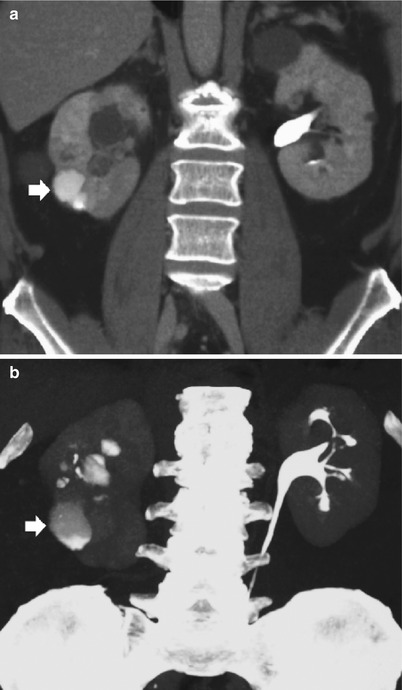
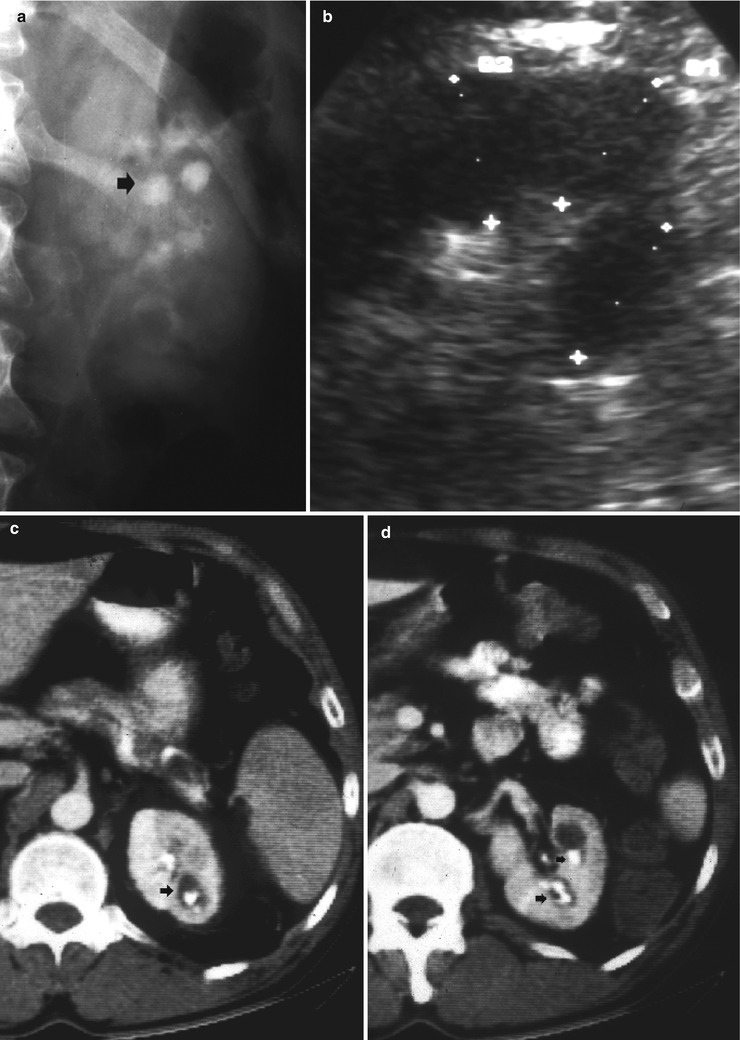
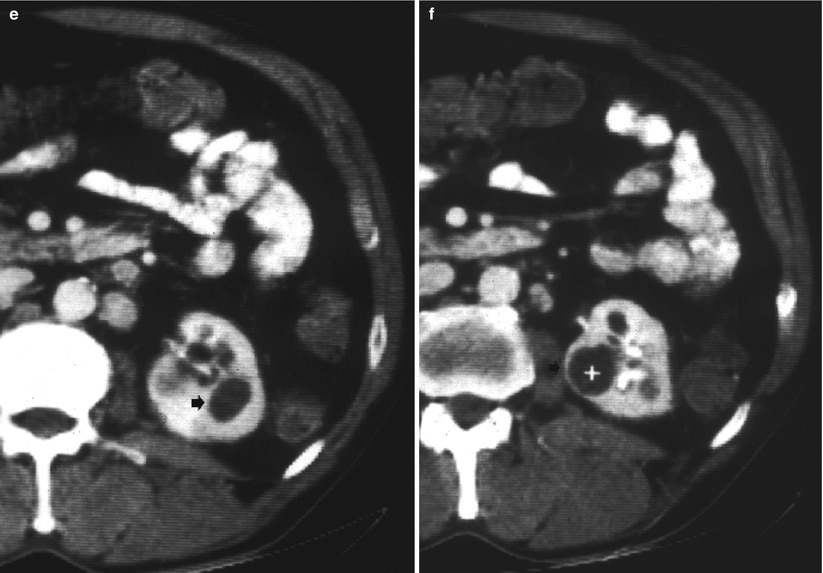
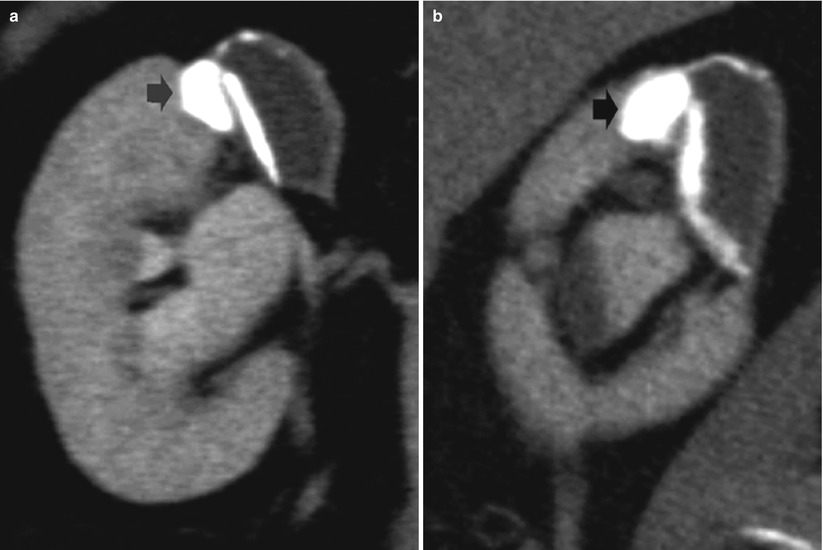
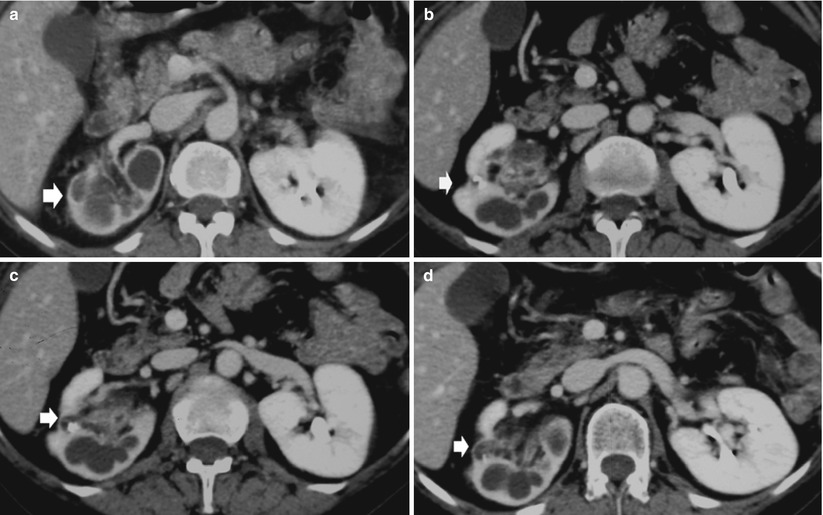
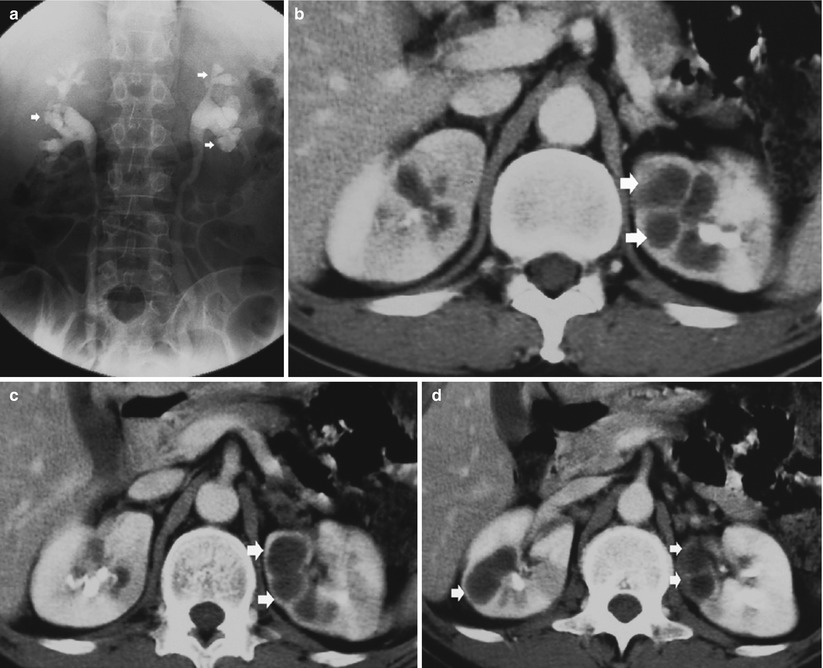

Fig. 10
Renal tuberculosis with caseified tissue necrosis. Open forms. The extensive cavitation of renal parenchyma results in noncommunicating (a) or communicating cavities (b) with the intrarenal excretory tract with deformation of the adjacent renal calyces from the simple narrowing of the calyx, medullary, and papillary necrosis, up to obstructive hydronephrosis

Fig. 11
Evolution of the open forms of renal tuberculosis. The caseified tissue necrosis (a) progressively spreads to the renal urinary tract (b). The progressive fibrosclerosis results in a noncommunicating cavity (c)
Fig. 12
Open or extensive form. Intravenous excretory urography–nephrotomography with cavitation (arrow) and extension of the caseified tissue necrosis to the intrarenal excretory tract

Fig. 13
(a) CT urography, coronal reformation. (b) Maximum intensity projection. A parenchymal cavity (arrow) communicating with the renal excretory tract is visualized on the right kidney


Fig. 14
Diffuse cavitating open form of renal tuberculosis. (a) Intravenous urography, and (b) grayscale ultrasound. Evidence of multiple cavities (arrow) on the upper pole of the left kidney communicating with the intrarenal collecting system. (c–f) Contrast-enhanced CT during the excretory phase. The left kidney shows diffuse parenchymal cavitation with resulting communicating (arrows in c and d) and noncommunicating cavities (arrow and caliper in e and f)

Fig. 15
(a, b) Renal tuberculosis. CT urography. Coronal (a) and sagittal (b) reformations of the right kidney. Fibrosclerosing tuberculosis with gross calcification (arrow) at the level of the renal parenchyma and adjacent noncommunicating cavity

Fig. 16
(a–d) Open form of renal tuberculosis with diffusion into the intrarenal collecting system extensive cavitation and fibrosclerosis with resulting noncommunicating cavities. Contrast-enhanced CT during the excretory phase. Extension of the caseified tissue necrosis to the intrarenal excretory tract with parenchymal fibrosclerosis with calcifications and irregularities of the renal margins (arrow) and resulting noncommunicating cavities

Fig. 17
Open form with diffusion into the intrarenal collecting system extensive cavitation and fibrosclerosis with resulting noncommunicating cavities. (a) Intravenous excretory urography. Irregularities in the morphology and deformations of the renal calyces (arrows). (b–d) Contrast-enhanced CT during the excretory phase. Renal parenchymal extensive necrosis with noncommunicating cavities (arrows) in both kidneys and parenchymal fibrosclerosis with calcifications and irregularities of the renal margins
The closed or fibrosclerotic form (Fig. 18) presents a better outcome to therapy and consists in the extension of the caseified necrosis toward the renal parenchyma. The host’s healing response induces fibrosis with calcium deposition, focal fibrosis with progressive parenchymal scarring, stricture formation with dilatation of the intrarenal urinary tract, and autonephrectomy (no functional contrast excretion). The fibrosclerotic forms of renal TB may appear as (1) pure fibrosclerosis with parenchymal scar (Fig. 19) often with evidence of noncommunicating cavities (Fig. 20) and (2) reactivation of the granulomatous process over a permanent status of fibrosclerosis with caseous necrosis and cavitation, or mixed fibrosclerotic and cavitating form, and resulting communicating or noncommunicating cavities with the intrarenal urinary tract (Fig. 21). Both forms determine parenchymal calcifications, deformation of the adjacent renal calyces from the simple narrowing of the calyx to medullary and papillary necrosis to obstructive hydronephrosis or hydrocalyx.
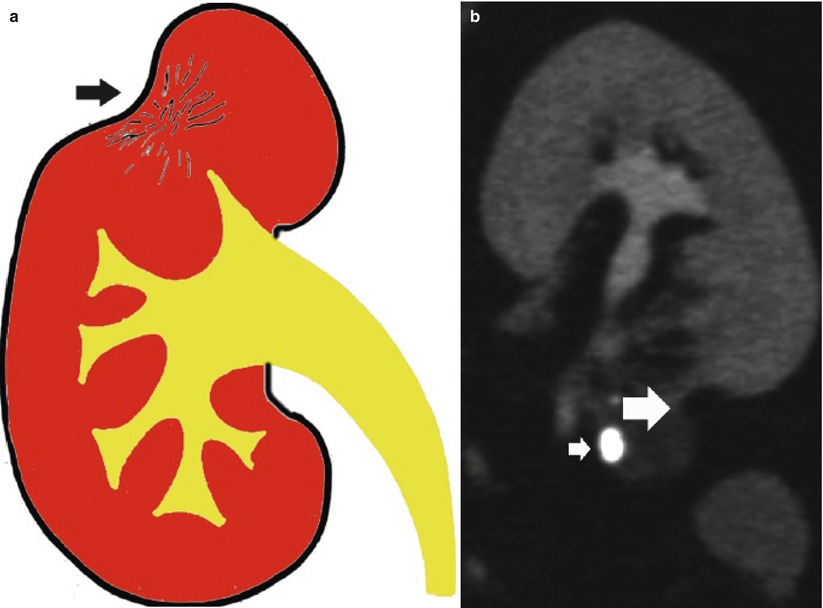
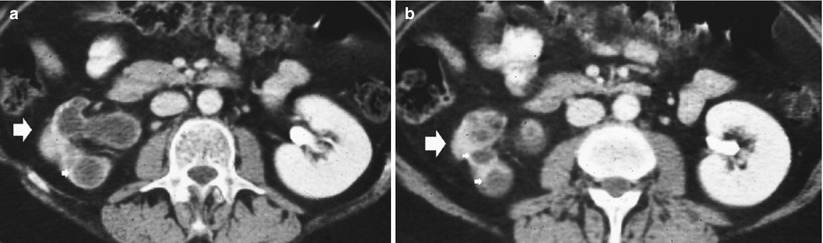





Fig. 18
(a) Scheme. Closed form of renal tuberculosis. The pathological process extends toward the renal parenchyma with progressive fibrosclerosis (arrow) and distortion of the adjacent renal calyces. (b) CT urography. Typical parenchymal fibrosclerosis (large arrow) with adjacent gross parenchymal calcification (small arrow)

Fig. 19
(a, b) Closed form of renal tuberculosis. Contrast-enhanced CT during the excretory phase. The right kidney shows extensive parenchymal fibrosclerosis (large arrows) with scar, dilatation of the intrarenal excretory tract (small arrows), and loss of excretory function with autonephrectomy (no contrast excretion is evident)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree