Fig. 1
Successful radio-frequency ablation of renal cell carcinoma. (a) Noncontrast CT demonstrates exophytic 2.5-cm tumor (arrow); (b) positioning of RF applicator with tip at distal end of tumor (arrow); (c) gas (arrows) is produced during ablation secondary to high-temperature coagulation; (d) immediate contrast-enhanced CT demonstrates nonenhancement of the tumor with a small surrounding “margin” of nonenhancement of the adjacent kidney (arrows) and small clinically insignificant perinephric hemorrhage (arrowhead); (e) 2 years after ablation, contrast-enhanced CT follow-up demonstrates continued nonenhancement of the tumor with a characteristic fat “halo” in the perirenal fat (arrowheads), suggesting complete treatment
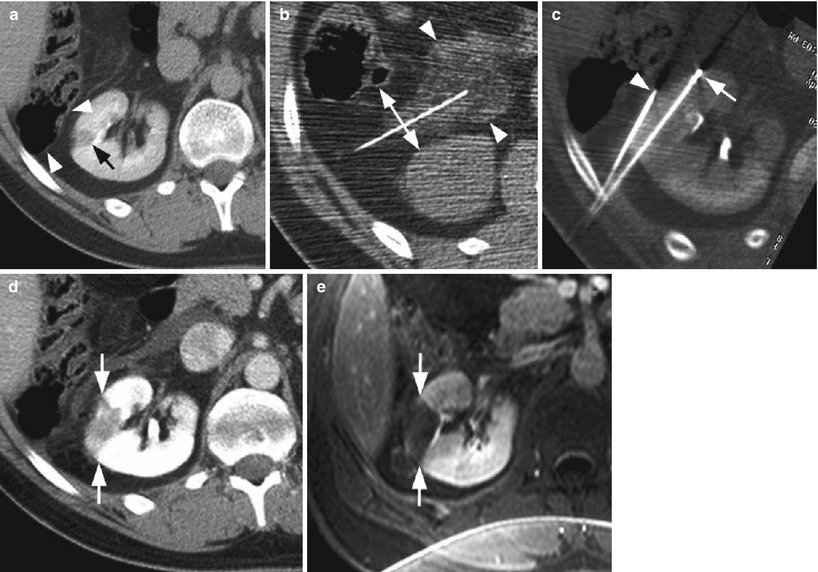
Fig. 2
The use of hydrodissection in thermal ablation to protect adjacent structures in a patient with multiple RCCs. (a) The colon (arrowheads) is closely approximated to the 1.3-cm tumor in the right kidney (black arrow), risking thermal injury; (b) 5 % dextrose in water (arrowheads) is injected into the perirenal fat which successfully separates the colon from the kidney (double–headed arrow); (c) applicator needle (arrow) is positioned into the tumor with the aid of a guiding needle (arrowhead); (d) immediate postablation CT demonstrates the ablative zone about the tumor (arrows); (e) 6-month follow-up MR with gadolinium demonstrates no recurrent tumor enhancement (arrows). A second 1.5-cm RCC in the right lower pole was also successfully treated during the same session (not shown)
Follow-up imaging also commonly demonstrates inflammatory stranding within the surrounding perirenal fat which should not be confused with residual tumor. Over time, a thin soft tissue halo may also appear within the surrounding fat due to encapsulation of fat necrosis which should not be interpreted as recurrent tumor (Fig. 1) (Gervais et al. 2003). More recently, it has also been observed that enhancing inflammatory nodules do rarely appear (<2 % of the time) after percutaneous ablation which may mimic tumor seeding of the applicator tract (Lokken et al. 2007). Real tumor tract seeding is exceeding rare and has only ever been reported once after radio-frequency ablation of RCC (Mayo-Smith et al. 2003). Instead, a new enhancing nodule within or adjacent to the applicator track is more likely to represent chronic inflammation containing histiocytes, granulation tissue, and fibrosis. These will usually appear as either a ring-enhancing nodule or ill-defined tram-tracking enhancement appearing 3–52 months after ablation (Lokken et al. 2007). Nodular enhancement along the ablated tumor margin, however, should be treated with suspicion for recurrence.
4.3 Outcomes
Results vary depending upon the modality (cryoablation vs. radio frequency) and applicator type (single vs. multitine), but meta-analyses across all percutaneous approaches yield a secondary effectiveness rate (i.e., no evidence of recurrence after multiple treatments is necessary) greater than 90 % for tumors smaller than 4 cm which is not significantly different than surgical treatment at 1 year (Hui et al. 2008).
4.3.1 Outcomes for Radio-frequency Ablation
Some midterm data is becoming available demonstrating recurrence-free survival rate of approximately 90 % at 5 years for tumors smaller than 4 cm (Levinson et al. 2008). Additionally, as technology and the learning curve have markedly progressed since treatment was performed for these initial survival data, future studies are expected to be as good or better. Again, treatment success is dependent upon size and location (exophytic vs. central) with near 100 % recurrence-free disease possible with selected tumor sizes (<4 cm) with larger tumors associated with increased risk of recurrence.
4.3.2 Outcomes for Cryoablation
Data is very limited for percutaneous cryoablation (Fig. 3), but short-term success (1 year) also appears to be excellent with success rates consistently above 95 % (Atwell et al. 2008). In addition, technical success was achieved with tumors ranging up to 7 cm, though the same principle of selecting tumors less than 4 cm still applies for optimal results (Stein and Kaouk 2007). Again, 5- and 10-year follow-up outcome data will be helpful with the caveat that current treatments will likely be superior given continuously technological improvements.
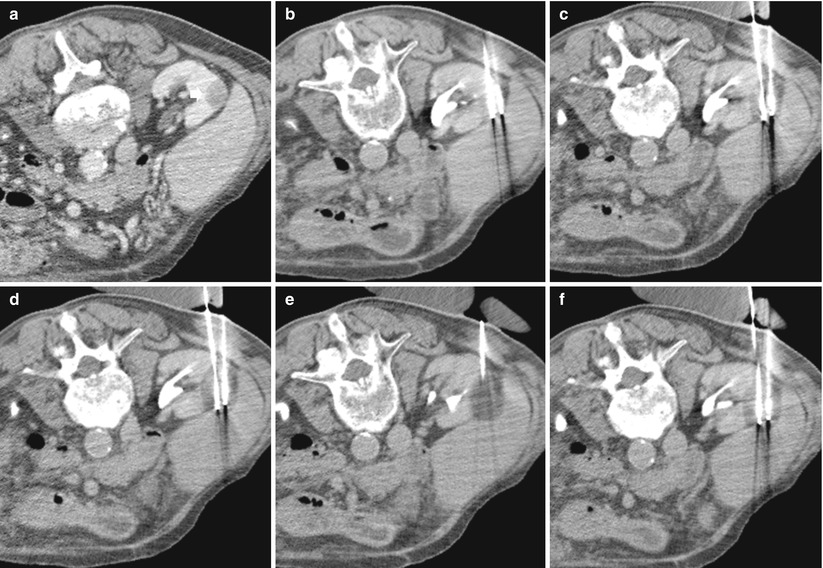

Fig. 3
The use of cryoablation in a patient with papillary RCC. (a) A small hypodense solid renal tumor (arrow) is evident in the right kidney (patient in prone position); (b) two needles are inserted in the mass. (c–f) progressive increase of the ice ball after two cycles of freezing and thawing
When comparing the outcomes of laparoscopic to percutaneous cryoablation at the same center, the procedural outcomes are demonstrably superior for percutaneous cryoablation including lower complications and transfusions (22 % vs. 40 %, respectively), shorter hospital stays (1.3 vs. 3.1 days, respectively), and lower narcotic use (5.1 vs. 17.8 mg, respectively). It should be noted that the complication rates reported in this series were higher than the accepted complication rate for percutaneous cryoablation (around 3 %) (Hui et al. 2008). Regardless, in short-term follow-up (13 months), cancer-specific survival is similar for percutaneous and laparoscopic cryoablation (100 and 100 %, respectively), and initial treatment failure was also not significantly different at 5.3 % (1/19) and 4.3 % (1/24), respectively.
4.4 Complications
The average complication rate is less than 5 % for both radio frequency and cryoablation, but almost none result in long-term morbidity. Radio-frequency ablation and cryoablation are both effective in the treatment of renal masses measuring 3 cm or smaller (Atwell et al. 2013). Major complications with either procedure are infrequent. Meta-analyses demonstrate a major complication rate of 3 % for percutaneous treatment vs. 7 % in the surgical treatment group (7 %; p < 0.05) which is the accepted clinical understanding (Hui et al. 2008; Johnson et al. 2004). The most common complications include perinephric hematoma, pneumothorax, nerve injury, and pain. Central tumors and tumors within the lower pole also run the risk of ureteral or ureteropelvic injury. A few case reports have documented nephrectomies that were necessary after ureteral injury or obstruction, but again, this is compared to oncologic treatment that could have included nephrectomy itself.
It is also important to note that the very low complication rate associated with RF ablation is reported in patients who were already deemed too high risk for surgical intervention because of advanced age or medical comorbidities. Thus, even in high-risk patients, percutaneous tumor ablation is associated with a very low complication rate.
5 Conclusion
Minimally invasive treatments for renal cell carcinoma such as percutaneous tumor ablation will undoubtedly become more prevalent as outcomes continue to be favorable and should be considered a viable treatment option for selected patients. Currently, the most common indications are elderly patients with small incidental tumors of unclear lethal potential and all high-risk surgical patients as this procedure has been shown to be safe in patients with multiple comorbidities. Multiple modalities are available, but the most common are RF and cryoablation which are likely similar in efficacy but still lack sufficient long-term data. These technologies are continuously improving, and it is expected that, as a result, patient selection and satisfaction will continue to expand.
References
Ahmed M, Liu Z, Afzal KS et al (2004) Radiofrequency ablation: effect of surrounding tissue composition on coagulation necrosis in a canine tumor model. Radiology 230(3):761–767PubMed
Ahmed M, Liu Z, Humphries S et al (2008) Computer modeling of the combined effects of perfusion, electrical conductivity, and thermal conductivity on tissue heating patterns in radiofrequency tumor ablation. Int J Hyperthermia 24(7):577–588PubMed
Ahrar K, Matin S, Wood CG et al (2005) Percutaneous radiofrequency ablation of renal tumors: technique, complications, and outcomes. J Vasc Interv Radiol 16:679–688PubMed
Atwell TD, Farrell MA, Callstrom MR et al (2007) Percutaneous cryoablation of large renal masses: technical feasibility and short-term outcome. AJR Am J Roentgenol 188(5):1195–1200PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree