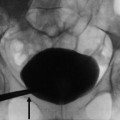
Fig. 1
(a–d) Scheme. Hydronephrosis grading. (a) First grade of hydronephrosis. Mild dilatation of the intrarenal urinary tract; (b) second grade of hydronephrosis with mild pyelocaliectasis with renal calyces maintaining the normal morphology; (c) third grade with pyelocaliectasis and renal calyces with a balloon shape; (d) fourth grade with a progressive thinning of the renal parenchyma and renal calyces which are eventually assimilated in one single sac
During obstruction, the kidneys undergo functional and biochemical alterations. Acute ureteral obstruction leads to a decrease in the renal blood flow and glomerular filtration rate, ultimately resulting in interstitial fibrosis and irreversible renal damage if untreated (Vaughan et al. 2004). Renal damage is initiated by high intratubular pressure, with initial renal vasodilatation followed by progressive vasoconstriction and decrease in renal blood flow, cellular atrophy, and necrosis. In addition, parenchymal infiltration by macrophages and T lymphocytes may cause scarring of the kidney (Klahr 1992). The rise in the intratubular pressure decreases the net hydrostatic filtration pressure across glomerular capillaries, resulting in a progressive decrease of GFR.
Since the consequences of obstructive uropathy are potentially reversible, prompt diagnosis and appropriate treatment are essential to prevent the permanent loss of renal function, which is directly related to the degree and duration of the obstruction. The site of obstruction determines the approach in these patients. If the obstruction is distal to the bladder, the placement of urethral catheter may be sufficient. Sometimes a suprapubic cystostomy is required. If the obstruction is located in the upper urinary tract, placement of percutaneous nephrostomy or passage of a retrograde stent may be necessary. Anyway, relief of obstruction at any stage is frequently followed by renal shrinkage with a variable pattern of postobstructive atrophy of the renal parenchyma. In patients with sepsis, appropriate antibiotic therapy is also indicated, while in some patients with acute renal failure, dialysis may be required (Klahr 1992). Surgical intervention can be delayed in low-grade or partial chronic urinary tract obstruction. Prompt relief of obstruction is indicated when there are repeated episodes of urinary tract infections, the patient has significant symptoms, there is urinary retention, or there is evidence of recurrent or progressive renal damage (Klahr 1992).
1 Obstructive Uropathy
1.1 Imaging Findings
The first imaging examination to be employed in obstructive uropathy is ultrasound (US). Multidetector computed tomography (CT) urography is currently thought to be the most sensitive and comprehensive imaging examination for the evaluation of the entire urinary tract and also in the assessment of obstructive uropathy (Caoili et al. 2002). Magnetic resonance (MR) urography is employed in the assessment of the morphology of the dilated urinary tract (Nolte-Ernsting et al. 2003), while MR excretory urography includes a dynamic functional study and it is useful in the functional assessment of the obstructed kidney (Teh et al. 2003). Even though several authors (Rohrschneider et al. 2002; Teh et al. 2003; Lefort et al. 2006) have shown substantial agreement between dynamic MR renography and radioisotope renography in renal function and excretion, technetium-99 m diethylenetriamine pentaacetic acid or MAG3-technetium-99 m diuretic renography remains the reference standard in the assessment of the renal function in renal tract obstruction.
1.1.1 Ultrasound
US is the most sensitive imaging technique in detecting renal hydronephrosis. US is accurate in detecting and assessing the grade of hydronephrosis (Fig. 2), even though it may reveal false-negative results, such as renal obstruction without dilatation (early obstruction, hypovolemia and dehydration, retroperitoneal disease), or false-positive results such as dilatation of the urinary tract in nonobstructed patients (extrarenal pelvis, parapelvic cysts, prominent renal vasculature, postobstructive dilatation, ureterovesical reflux, and congenital megacalyces). There is no correlation between the grade of hydronephrosis and the grade of obstruction. Nowadays, the employment of microbubble-based contrast agents allows an accurate assessment of renal parenchyma perfusion (Quaia et al. 2009) which can be reduced in the acute or chronic urinary tract obstruction.
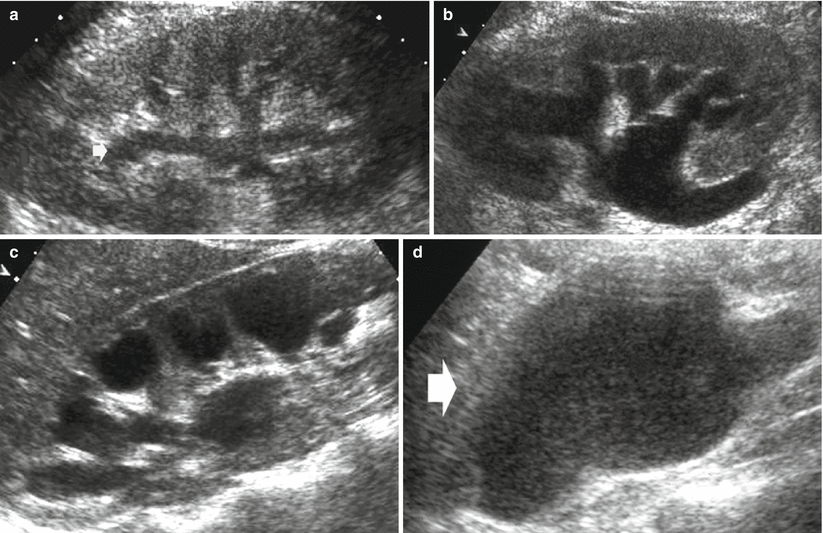

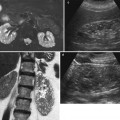
Fig. 2
(a–d) Ultrasound, longitudinal scan. Hydronephrosis grading. Progressive dilatation of the intrarenal collecting system and pyelocaliectasis with a progressive reduction of the renal cortical thickness. (a) First grade of hydronephrosis with mild dilatation of the intrarenal urinary tract (arrow). (b) Second grade of hydronephrosis with pyelocaliectasis and normal morphology of the renal calyx. (c) Third grade of hydronephrosis with pyelocaliectasis and renal calyces with a balloon shape. (d) Fourth grade of hydronephrosis with a progressive thinning of the renal parenchyma (arrow)
Renal vasoconstriction is the key factor in the pathophysiological course of acute and chronic obstruction and increased RI values result. Resistive index (RI) assessment is a separate and distinct parameter from collecting system dilatation to assess urinary obstruction (Platt 1996, Platt et al. 1993). Duplex Doppler sonography has been reported to be a useful noninvasive imaging method for the diagnosis of renal obstruction. RI elevation (Fig. 3) occurs by 6 h of clinical acute renal obstruction and may precede pyelocaliectasis. A mean RI >0.7, a difference >0.06–0.08 between the mean RI of the two kidneys, and a progressive increase in RI following fluid administration and diuretics have been considered diagnostic of obstruction (Platt 1992; Quaia and Bertolotto 2002). A significant fall in RI values is reported after nephrostomy. In general, there should be a rapid return to normal RIs if obstruction is relieved within 5 h, whereas RIs may take days or even weeks to return to baseline levels if obstruction is present for at least 18–24 h.
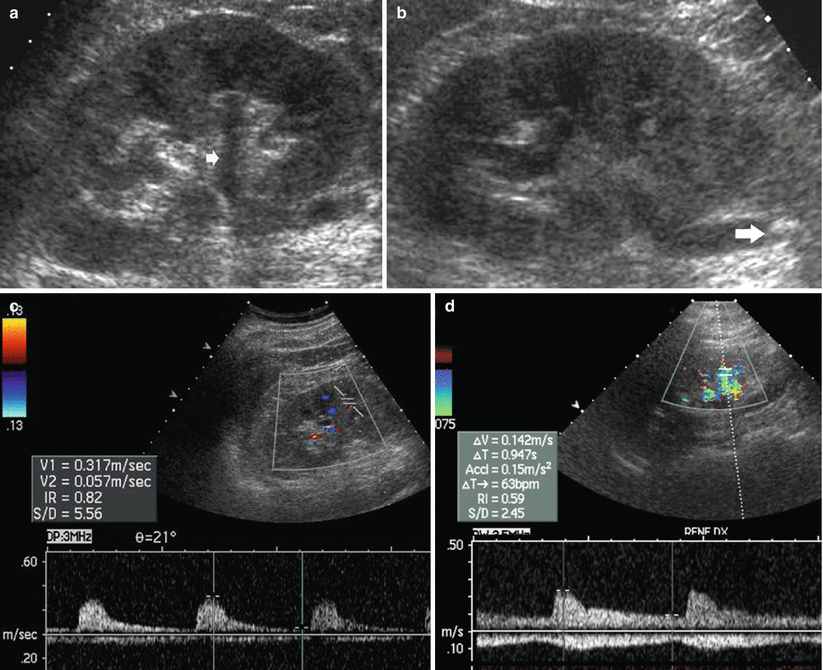

Fig. 3
(a–d) Urinary tract obstruction due to ureteral stone. (a) US longitudinal scan of the right kidney revealing a mild pyelocaliectasis (arrow) with a normal morphology of the renal calyces and normal thickness and dimension of the renal parenchyma; (b) renal obstruction is caused by a stone (arrow) in the proximal part of the ureter; (c) the resistive index is increased (0.82) in the segmental arteries of the obstructed kidney, while it is normal (0.59) in the contralateral kidney (d)
The evaluation of renal parenchyma RIs presents a controversial role in acute renal colic with a sensitivity from 19 to 92 % and a specificity from 88 to 98 % according to the different series (Platt et al. 1989; Tublin et al. 1994; Lee et al. 1996). This is determined by the absence of a true reference standard to diagnose renal obstruction and by the possible presence of partial and intermittent obstruction. Normal RI likely reflects the lack of obstruction of sufficient degree to produce vasoconstriction (Platt 1996).
1.1.2 Unenhanced Spiral CT
Unenhanced spiral or multidetector-row CT is now considered the reference imaging technique for one specific form of urinary obstruction, namely, urolithiasis (see chapter “Nephrocalcinosis and nephrolithiasis”). The direct finding is the visualization of the calculus itself. Indirect signs include renal pelvis and ureteral dilatation (frequency, 65–90 %), perinephric and periureteral stranding (36–82 %), rim sign around the ureter (frequency 50–77 %), renal enlargement (frequency 36–71 %), renal sinus fat blurring, and decreased attenuation of the renal parenchyma (Smith et al. 1995; Niall et al. 1999). It has to be underlined that the reduced attenuation of the renal parenchyma is not specific for the renal colic since it may be caused also by interstitial edema in acute pyelonephritis and by venous congestion in renal vein thrombosis.
1.1.3 Intravenous Excretory Urography and CT Urography
Before the advent of helical CT and CT urography, intravenous excretory urography was indicated in the diagnosis of renal hydronephrosis, in the identification of the stone, in the differentiation from other calcific densities, and to provide functional informations about the kidney. Even until the late 1990s, intravenous excretory urography because of its superior spatial resolution was still considered the examination of choice for evaluating the urothelium (Silverman et al. 2009). Intravenous excretory urography presents a detection rate of urinary stones ranging from 70 to 90 % (Miller et al. 1998). Intravenous excretory urography reveals the grade and extent of dilatation of the urinary collecting system proximal to obstruction with the evidence of the site of obstruction. In acute renal obstruction, the classic urographic features are the dimensional increase of the kidney, increasing nephrographic density defined as obstructive nephrogram, and delayed contrast excretion with delayed calyceal appearance on excretory urography (Fig. 4) also with variable dilatation of the collecting system and ureter (Talner et al. 2000a). Dilatation is often mild or lacking in acute obstructive uropathy, even when obstruction is complete. Anyway, intravenous excretory urography is a time-consuming imaging technique especially in obstructed patients, and it implied high radiation dose and iodinated contrast agent administration. Moreover, intravenous excretory urography is not advocated in acute ureteral obstruction due to iodinated contrast – induced diuresis.
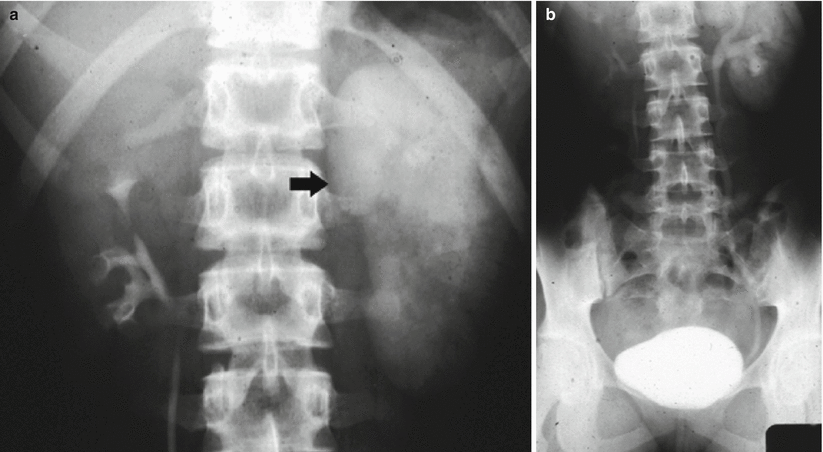

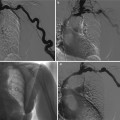
Fig. 4
(a, b) Acute urinary tract obstruction due to a urinary stone located in the intramural tract of the left ureter. Intravenous excretory urography. (a) Increasing nephrographic density on the left kidney (arrow) with delayed contrast excretion (b). Renal calyces appeared 1 h after iodinated contrast injection also with evidence of dilatation of the collecting system and ureter
On CT urography, it is difficult to obtain a single image of all urinary collecting system segments in an opacified and distended state. Furosemide improves the urinary tract and ureteral opacification and distension (Silverman et al. 2006) with a greater effect than saline by increasing the urinary flow rate and the delivery of contrast medium to the middle and distal ureter. Postprocessing techniques are similar in most centers. A combination of rendering techniques has been used, including multiplanar reformat (MPR), curved planar reformat (CPR), thin-slab or thick-slab average intensity projection (AIP), volume rendering (VR), or maximum intensity projection (MIP) 3D images.
The urographic signs of acute and chronic obstruction have their counterparts on CT urography. CT urography reveals the grade and extent of dilatation of the urinary collecting system proximal to obstruction with the evidence of the site of obstruction (Fig. 5). The obstructed kidney swells slightly because of enlargement of the tubules and interstitial edema. In acute renal obstruction, the classic urographic features are an increasing nephrographic density defined as obstructive nephrogram and delayed contrast excretion with delayed calyceal appearance on excretory urography and also with variable dilatation of the collecting system and ureter (Talner et al. 2000a). Dilatation is often mild or lacking in acute obstructive uropathy, even when obstruction is complete.
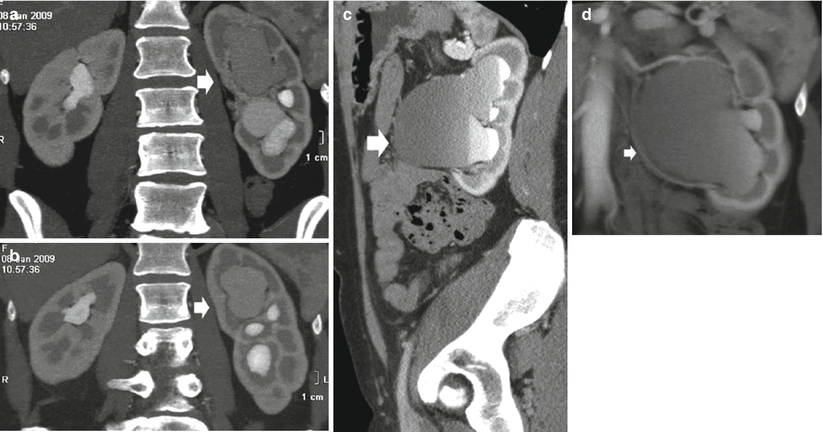

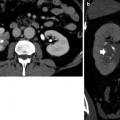
Fig. 5
(a–d) Ureteropelvic junction obstruction in a 21-year-old woman due to an arterial crossing vessel (accessory renal artery). (a, b) Multidetector CT urography; (c) curved multiplanar reformation; dilatation of the left intrarenal collecting system (arrow) with reduction of renal cortex thickness due to chronic obstruction. (d) 3D maximum intensity projection. Evidence of the crossing vessel (arrow) determining the ureteropelvic junction obstruction
Chronically obstructed kidneys may be small, normal, or large. The volume is determined by many factors, the most important being the duration and degree of obstruction and the distensibility of the collecting system (Talner et al. 2000a). The obstruction of the urinary tract, if not treated, usually determines a progressive atrophy of the renal parenchyma. In postobstructive renal atrophy, renal medulla and cortex are both reduced in their thickness (Fig. 5). Chronic obstructive uropathy may also present a nondilated renal collecting system in 4–6 % of cases (Naidich et al. 1986). This may be determined by the tumoral infiltration of the ureteral wall or by chronic incomplete obstruction of the ureter which may be suddenly complicated by an acute event such as infection.
Most of the false-positive findings of US, including extrarenal pelvis and parapelvic cysts simulating urinary tract obstruction, are easily differentiated from true obstruction by CT urography (Fig. 6).
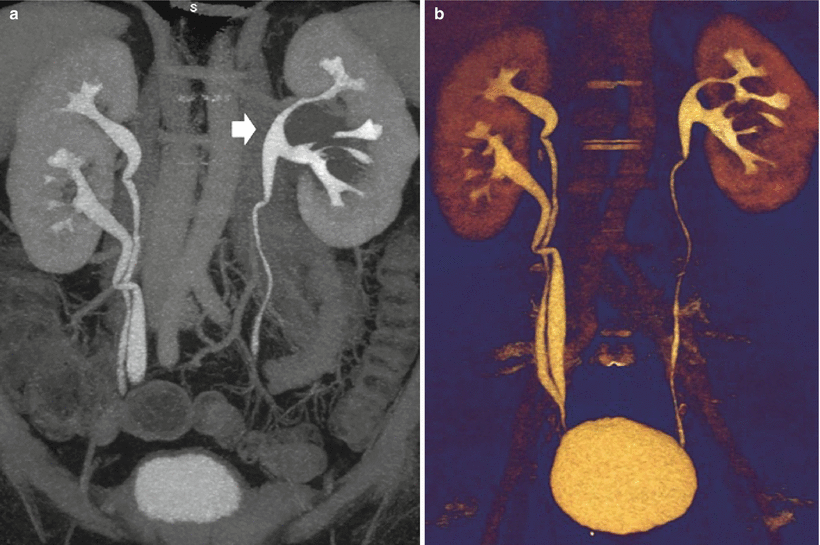

Fig. 6
(a, b) Parapyelic cysts simulating urinary tract obstruction. (a) 3D maximum intensity projection depicts the spatial morphology and the relationship between the cysts and the renal calyces. (b) Color-coded volume rendering (VR) confirms calyceal distortion
1.1.4 MR Urography
MR urography has received a relatively lower attention than multidetector CT urography, being hampered by the low spatial resolution, which is crucial for calyceal evaluation and by the requirement of updated MR units. The use of a phased array body coil is preferable because of the improved signal-to-noise ratio (SNR). Currently 1–1.5-T systems are generally used for abdominal imaging, but the advent of 3T MRI systems brings a twofold increase in the SNR. The increase in SNR can be spent on higher resolution or on even faster imaging. When combined with parallel imaging techniques such as sensitivity encoding (SENSE), the speed of any sequence can be increased by up to a factor of four or higher.
Excellent contrast resolution and lack of ionizing radiation make MR urography a useful technique for noninvasively evaluating the entire urinary tract, especially when ionizing radiation is to be avoided, such as in pediatric or pregnant patients. A number of studies have shown that MR urography is a useful examination for investigating the causes of urinary tract dilatation and diagnosing urinary tract obstruction in adult and pediatric populations (Roy et al. 1998; Shokeir et al. 2004; Kocaoglu et al. 2005). Fast imaging techniques are essential because of respiratory motion of the kidneys, and MR scan should be performed within one breath-hold according to patient compliance. The patient should get clear instructions on breath-hold technique. If the patient has difficulty with breath-holding, a short period of hyperventilation before breath-holding may be helpful. If the sequence is too long to perform in one breath-hold, respiratory triggering can be used. Another technique of respiratory motion compensation control is respiratory gating by use of a navigator pulse. Breath-hold imaging at the end of expiration should be performed in compliant patients, while nonbreath-hold imaging should be performed in noncompliant patients. Nonbreath-hold imaging should include single-shot sequences – magnetization-prepared gradient echo, and half-Fourier single-shot technique (HASTE) providing a shorter echo time, poorer signal-to-noise ratio, and reduced contrast-to-noise ratio if compared to TSE T2-weighted sequence – respiratory gating or respiratory triggeringor MR imaging navigators.
The static-fluid MR urography utilizes unenhanced, heavily T2-weighted pulse sequences to image the urinary tract (Nikken and Krestin 2007) including half-Fourier single-shot echo-train spin-echo sequences (such as HASTE), thick-slab RARE, or respiratory-triggered three-dimensional echo-train spin-echo sequences. On the heavily T2-weighted images, the urinary tract is hyperintense because of its long relaxation time, independent of the excretory renal function (Fig. 7). Additional spectral fat suppression can help further improve the contrast between the urinary tract fluid and retroperitoneum. Static-fluid MR urography sequences are typically used in the coronal plane, although other imaging planes can be used. Two fundamental techniques may be employed: (1) single-slice projection images in which a single 6–8 cm slice is employed and (2) multislice technique employing sequential static-fluid acquisitions by a 3D multiple slice technique with longer acquisition time to ensure visualization of the entire ureters and assess for a fixed narrowing or obstruction. This technique is ideally suited for patients with dilated or obstructed collecting system.
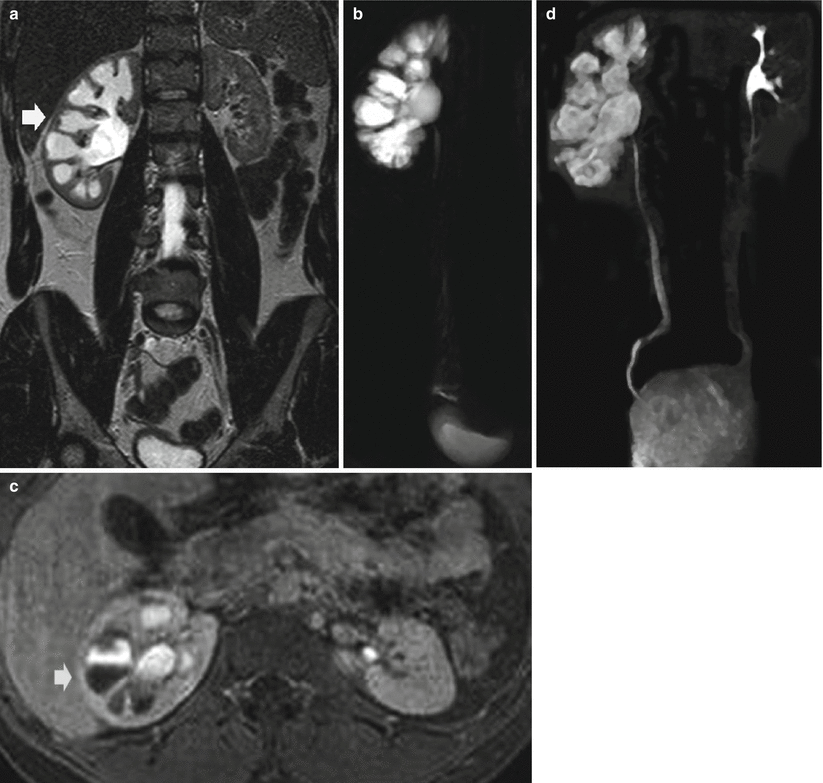

Fig. 7
(a–d) Urinary tract obstruction due to ureteropelvic junction obstruction. (a) T2-weighted turbo spin-echo sequence. Coronal plane. (b) Static-fluid MR urography. Dilatation of the right intrarenal collecting system (arrow in a) with reduction of renal cortex thickness due to chronic obstruction. (c) T1-weighted 3D gradient-echo MR sequence. Excretory phase after gadolinium injection. Dilatation of the right intrarenal collecting system (arrow) with contrast excretion and evidence of the T2* effect gadolinium with reduction of urine signal intensity. (d) Excretory MR urography, 5 min after gadolinium injection. Maximum intensity projection 3D image. Dilatation of the right intrarenal collecting system with clear visualization of the intrarenal and extrarenal urinary tract
The advantages of single-slice projection are the short acquisition time (6–8 s), absence of motion artifacts, no necessity of postprocessing, and the possibility to identify the location of obstruction. The main drawbacks of the single-slice technique are the low spatial resolution, the low SNR, and the impossibility to define the cause of obstruction. The advantages of multislice technique are reduced partial volume averaging artifact and the increased spatial and contrast resolution. The drawbacks of multislice technique are the longer acquisition time compared to the single-slice technique and the superimposing extraurinary fluid. When MR urography is combined with assessment of the renal parenchyma and soft tissues of the abdomen and pelvis, precontrast T1- and T2-weighted images on the axial or coronal plane of the abdomen and pelvis are obtained.
In excretory MR urography, intravenous gadolinium is combined with a T1-weighted 3D gradient-echo (GRE) sequence. Excretory MR urography provides high-quality images of both nondilated and obstructed collecting systems. When information about vascular structures is critical, such as the evaluation of pyeloureteral (ureteropelvic) junction obstruction in which a crossing vessel is suspected, an MR angiography-type sequence is performed in the coronal plane. Alternatively, a dynamic, axial, fat-suppressed sequence can be performed through the kidneys to capture the precontrast, corticomedullary, nephrographic, and excretory phases. Excretory phase images are then typically obtained approximately 5 min after intravenous gadolinium chelate administration at a dose of 0.05–0.1 mmol/kg and are usually accomplished with some type of fat-suppressed three-dimensional gradient-echo sequence. Having patients raise their arms over their heads during coronal acquisitions reduces wraparound artifact.
Excretory MR urography performed after intravenous gadolinium chelate administration without pharmacologic intervention is often lacking due to suboptimal ureteral distention, uneven distribution of the contrast material in the collecting systems, and the hypointense T2* effect of concentrated gadolinium (Hagspiel et al. 2005). Therefore, some combination of intravenous hydration and diuretic administration is typically used adjunctively (Karabacakoglu et al. 2004). Furosemide (0.05–0.1 mg/kg) is the diuretic typically used for excretory MR urography and can be used successfully in adults at doses as low as 5 mg. Oral hydration is discouraged, as the high T2 signal intensity of fluid within the bowel can interfere with the visualization of the urinary tract during static-fluid MR urography, particularly when maximum intensity projections are rendered.
For excretory MR urography, the thinnest partitions that maintain acceptable SNR and anatomic coverage should be prescribed. Breath-holding is essential for excretory phase imaging, as acquisition times of 20–30 s are typical with most widely available three-dimensional gradient-echo sequences. A through-plane resolution of 2–3 mm is usually achievable with currently available MR systems operating at 1.5–3.0 T. The use of parallel imaging techniques reduces acquisition times with a modest penalty in SNR. For patients with limited breath-holding capacity, the urinary tract can be imaged in segments. Static-fluid MR urography sequences should be performed prior to excretory MR urography sequences, as excreted gadolinium chelate can reduce the signal intensity of urine on T2-weighted images. Also, the bladder is best evaluated on T1-weighted images within the first few minutes after intravenous contrast material administration but before excreted gadolinium chelate reaches the bladder via the ureters.
By combining sequences designed to evaluate the collecting systems, ureters, and bladder with sequences designed to evaluate the kidneys (particularly for masses), a comprehensive assessment of the urinary tract became possible. The all-in-one technique includes SE T1-weighted, dual-echo GRE T1-weighted, TSE T2-weighted with fat suppression, diffusion sequences, static-fluid MR urography, and excretory MR urography. The general MR protocol consists of the following sequences: (1) coronal T2-weighted HASTE (TR infinite, TE 120 ms, flip angle 90°, breath-hold), serving as a localizer but also supplying valuable T2-weighted information. The limitation of this sequence is a relatively low SNR. (2) Transverse T2-weighted turbo spin-echo sequence with fat suppression (TR 2,000 ms, TE 100 ms, flip angle 90°, respiratory triggering). This sequence provides for more detailed T2-weighted information. (3) Transverse T1-weighted gradient-echo sequence, in-phase and opposed-phase (TR 180 ms, TE 2.3 ms/4.6 ms, flip angle 90°, breath-hold), preferably as a dual-echo sequence. (4) Static-fluid MR urography. (5) Transverse or coronal 3D T1-weighted fast spoiled gradient-echo sequence for dynamic imaging (TR 130 ms, TE 1.0 ms, flip angle 90°), using intravenous gadolinium contrast, immediately followed by three breath-hold periods with four scan series per breath-hold. In this way precontrast and postcontrast images in arterial, nephrographic, and excretory phase are obtained. (6) Coronal 3D fast gradient echo with fat suppression, obtained immediately after the dynamic series for delayed contrast-enhanced images (TR 3 ms, TE 2 ms, flip angle 15°) corresponding to the MR excretory urography.
1.2 Causes of Obstruction
1.2.1 Congenital Anomalies
Congenital anomalies of the renal collecting system are one of the principal causes of renal tract obstruction (Table 1). The most common obstruction of normally placed ureters in children occurs in the juxtavesical segment and is called primary obstructive megaureter.
Table 1
The different congenital and acquired causes of renal tract obstruction
Causes |
|---|
Congenital |
Hydrocalyxa |
Calyceal diverticulumb |
Ureteropelvic junction obstruction |
Ureteral obstruction (namely, primary obstructive megaureter) |
Urethral valves |
Ureterocele |
Acquired |
Scarring (inflammation, surgery, trauma) |
Intraluminal ureteral obstruction (stone, blood clot, sloughed papilla, tumors) |
Extraluminal ureteral obstruction |
Postsurgical ureteral obstruction |
Radiation ureteral stricture |
Retroperitoneal fibrosi |
Pelvic lipomatosis |
Pregnancy |
Gynecological causes (endometriosis, pelvic inflammatory disease) |
Gastrointestinal disease (Crohn’s disease, diverticulitis, acute pancreatitis, tumors) |
Benign prostatic hypertrophy and prostatic carcinoma |
Megapolycalicosis (Fig. 8) is defined as a congenital dilatation of the renal calyces without evidence of obstruction. In the absence of complications such as infection and stones, megapolycalicosis would not have a particular clinical relevance if not for the possible misinterpretation as hydronephrosis. Megaureter is frequently associated to megapolycalicosis. Postobstructive renal atrophy must be differentiated from megapolycalicosis and reflux nephropathy based on the ratio between the renal medulla and cortex. In normal cases, the ratio between the renal medulla and cortex is 1.5–2 to 1. In megapolycalicosis, the renal medulla presents a reduced thickness while the cortex is normal and the ratio medulla/cortex is near to 1.
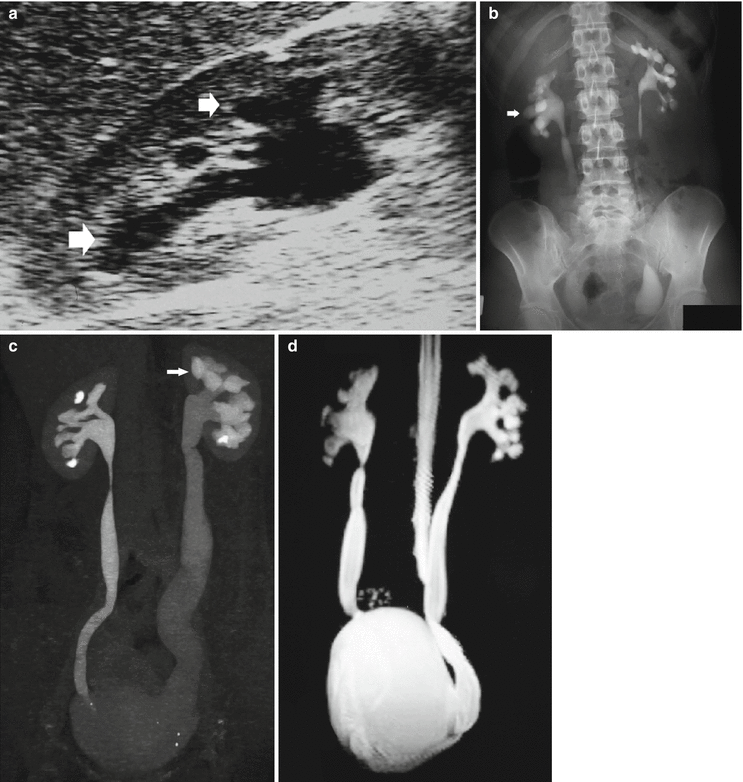

Fig. 8
(a–d) Congenital megaureter in a 22-year-old woman. (a) Ultrasound. Dilatation of the intrarenal collecting system (arrow). (b) Intravenous excretory urography; (c) CT urography; (d) static-fluid MR urography. Dilatation (megapolycalicosis) of the renal calyces (small arrows) in both kidneys and bilateral megaureter (large arrow) without any evidence of distal obstruction
Other causes of ureteral obstruction are ureteral valve, ureteral atresia, circumcaval (retrocaval, postcaval) ureter, and ureterocele. Ureterocele (associated with duplicated excretory system in 80 % of cases) is mainly asymptomatic, but it may cause obstruction, urinary stasis, reflux, and stone formation particularly if ectopic in location. An ectopically inserting ureter draining the upper moiety of a kidney with duplication of the urinary tract is frequently involved in obstruction.
1.2.2 Ureteropelvic Junction Obstruction
Ureteropelvic junction obstruction, also named pelviureteric junction obstruction or idiopathic, pelvic, or congenital hydronephrosis, is defined as an obstruction of the flow of urine from the renal pelvis to the proximal ureter with a consequent pressure increase in the renal pelvis. It represents a major cause of obstructive uropathy at all ages and is the most common cause of neonatal hydronephrosis (Talner et al. 2000b). This pathologic entity may have congenital or acquired causes and requires frequently a surgical treatment consisting of open or laparoscopic pyeloplasty or endopyelotomy. Pelvic kidneys are associated with an increased incidence of ureteropelvic junction obstruction and of stone formation. Other causes are reported in Table 2. It has been suggested that accessory renal arteries in the lower pole of either kidney that cross the ureteropelvic junction contribute to ureteropelvic junction obstruction in up to 11 % of children (Hoffer and Lebowitz 1985) and 52 % of adults (Lowe and Marshall 1984).
Table 2
The different congenital and acquired causes of ureteropelvic junction obstruction
Causes |
|---|
Congenital |
Anomalies of rotation |
Intrinsic stenosis |
Segment dysfunction (adynamic segment) |
Kinkings or angulations |
Adhesions or bands |
Crossing vessel (lower pole renal artery) |
Horseshoe kidney |
High insertion of the ureter |
Urethral valves |
Acquired |
Scarring (inflammation, surgery, trauma) |
Reflux |
Malignant tumors (transitional cell carcinoma, squamous cell carcinoma, metastasis) |
Benign tumors (polyps, mesodermal tumor) |
Intraluminal lesions (stone, clots, papilla, fungus ball, cholesteatoma) |
It is twice more frequent in the left than in the right kidney and occurs more frequently (about 90 % of cases) in kidneys with an extrarenal pelvis, and it is the most common form of urinary tract obstruction in pediatric patients. Unilateral obstruction is more frequent than bilateral obstruction (10–30 % of cases). It manifests with acute flank pain.
On US, the renal pelvis appears disproportionately large compared to dilated calyces. CT urography with multiplanar reformations (MPRs) is the most accurate imaging technique to identify the cause of ureteropelvic junction obstruction including crossing vessels. Pyelocaliectasis with ureteropelvic junction narrowing with normal-caliber ureter downstream is evident on imaging (Fig. 9). Ureteropelvic junction obstruction may be segmental involving the superior or inferior moiety in patients with duplication of the renal collecting system or with a bifid renal pelvis (Fig. 10). Long-standing ureteropelvic junction obstruction may manifest with severe hydronephrosis and marked parenchymal atrophy and minimal contrast excretion (Fig. 11).
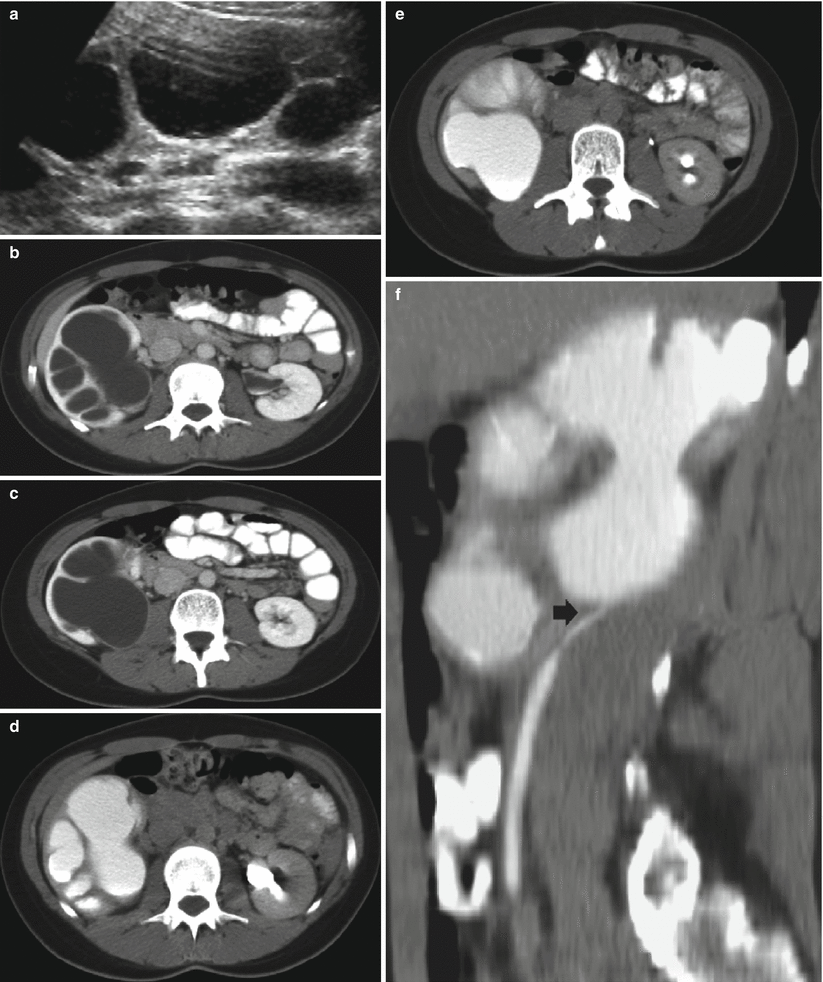
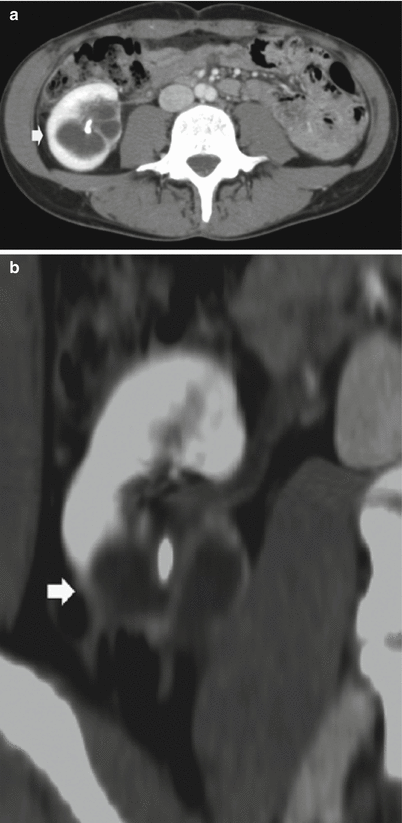
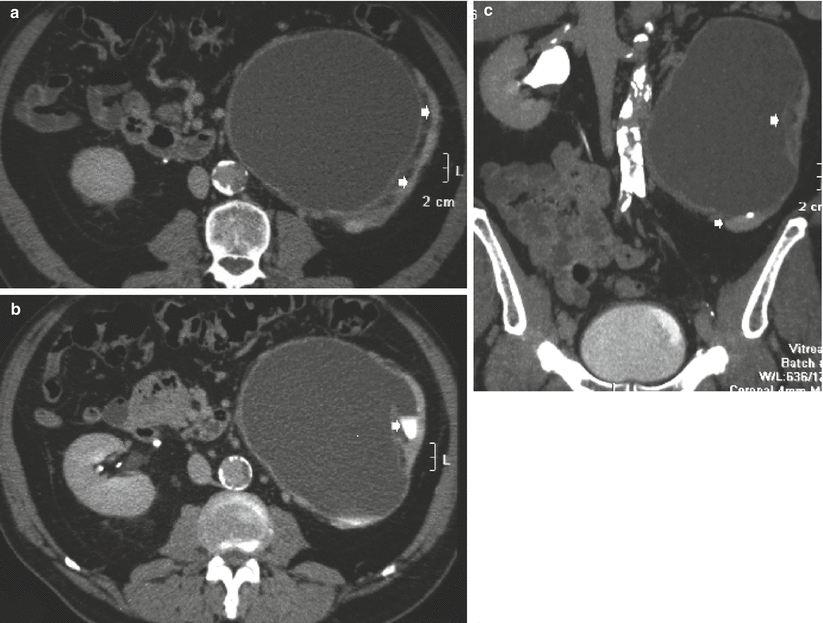

Fig. 9
(a–f) Ureteropelvic junction obstruction in a 21-year-old woman presenting with acute flank pain. (a) Ultrasound. Fourth-grade hydronephrosis of the right kidney. (b, c) Contrast-enhanced CT. Excretory phase. The right kidney presents a fourth-grade hydronephrosis with no evidence of contrast excretion due to urinary tract obstruction. (d, e) Delayed CT scan 20 min after iodinated contrast administration. (f) Coronal reformation of the scan obtained 20 min after contrast administration. Ureteropelvic junction obstruction with pyelocaliectasis and ureteropelvic junction narrowing (arrow) with normal-caliber ureter downstream

Fig. 10
(a, b) Segmental ureteropelvic junction obstruction in a 42-year-old female patient with bifid renal pelvis who had surgery for vesicoureteral reflux during childhood and presenting with acute flank pain. Contrast-enhanced CT, nephrographic phase. (a) Transverse plane. (b) Coronal reformation. Segmental dilatation of the intrarenal urinary tract due to surgical scarring of the ureteropelvic junction at the lower pole of the right kidney (arrow)

Fig. 11
(a–c) Long-standing ureteropelvic junction obstruction with chronic urinary tract obstruction. CT scans (a, b) and multiplanar reformat (c) showing the typical findings of long-standing ureteropelvic obstruction. There is severe hydronephrosis with marked parenchymal atrophy (arrow in a). Minimal contrast excretion is noted (arrow in b). Lower scan showed absence of ureteral dilatation
1.2.3 Urolithiasis and Acute Renal Colic
Renal colic refers to the passage of a renal calculus from the renal pelvis into the ureter characterized by acute pain, generally beginning in the flank and radiating into the groin (Andreoli 1992). Renal colic pain constitutes the most common cause of patients with an acute abdomen seen in emergency department. Renal colic due to stone obstructing the ureteropelvic junction, the pelvic brim where the ureter crosses the iliac vessels, or the ureterovesical junction represents the most common cause for acute renal obstruction, and 40–60 % of patients referred for CT for a suspicion of colic pain have an obstructing renal stone (Taourel et al. 2008).
Renal stones form by an initial crystallization of a nidus (nucleation) from a supersaturated urine with subsequent crystal growth and aggregation of the nidus into macroscopic stone (Pak 1992




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree