Contrast
Oral (750 mL of water) and intravenous contrast (120 mL)
Noncontrast images
ST: 5 mm
RT: 0.5 s
SA: Top of the liver to bottom of the kidneys
Corticomedullary phase
ST: 5 mm
RT: 0.5 s
RI: 2.5 mm
ScT: 30 s delay
SA: Top of liver to bottom of the kidneys
Nephrographic phase
ST: 5 mm
RT: 0.5 s
RI: 2.5 mm
ScT: 80 s delay
SA: Top of liver to symphysis pubis
Delayed phase
ST: 5 mm
RT: 0.5 s
RI: 2.5 mm
ScT: 5 min delay
SA: Top of liver to bottom of kidneys
Benign Renal Neoplasms
Benign renal neoplasms are classified according to the cell type of the tumor (Table 1.2).
Table 1.2
World Health Organization (WHO) classification of benign renal neoplasms
Renal cell tumors | Metanephric tumors | Mesenchymal tumors | Mixed epithelial and mesenchymal tumors |
|---|---|---|---|
Oncocytoma | Metanephric adenoma | Angiomyolipoma | Cystic nephroma |
Renal cortical (papillary) adenoma | Metanephric stromal tumor | Leiomyoma | Mixed epithelial and stromal tumor |
Metanephric adenofibroma | Hemangioma | ||
Lymphangioma | |||
Reninoma | |||
Fibroma | |||
Schwannoma | |||
Congenital mesoblastic nephroma |
Oncocytoma
General Information
Solid epithelial neoplasm.
Represents between 10 % and 15 % of small (<3 cm) solid renal neoplasms.
Typical presentation of oncocytoma is between sixth and seventh decades.
Incidence of bilateral oncocytoma is 5 %, and bilateral multifocal oncocytoma is <2 %.
Imaging
Ultrasound
Ultrasound is nonspecific and shows usually a smooth well-defined renal mass with mixed echogenicity (Fig. 1.1a, b).
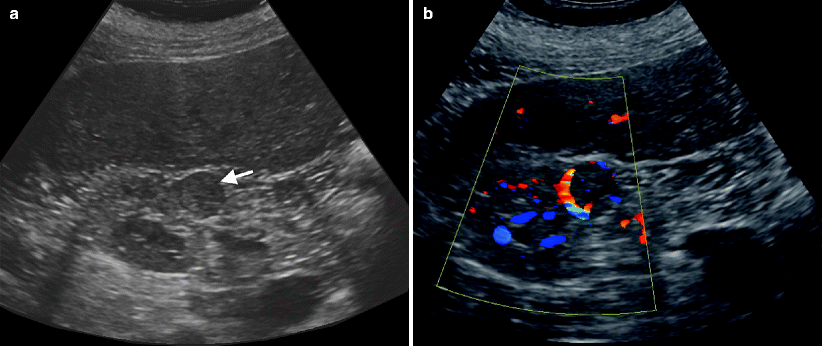
Fig. 1.1
Oncocytoma. (a) Gray-scale ultrasound demonstrates a well-defined mass (arrow) isoechoic to the renal parenchyma. (b) Color flow Doppler ultrasound reveals prominent peripheral and minimal internal blood flow
Computed Tomography
Well-defined mass with smooth, rounded margin.
Usually solitary, but may be multiple and/or bilateral.
Calcifications are rare.
Small oncocytomas are usually homogeneous in appearance on contrast-enhanced CT scans.
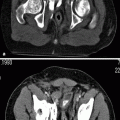
A central, sharply defined stellate scar is present in 25–33 % of large oncocytomas and is highly suggestive of the diagnosis (Fig. 1.2a). A central scar can also be seen in renal cell carcinoma, particularly chromophobe RCC.
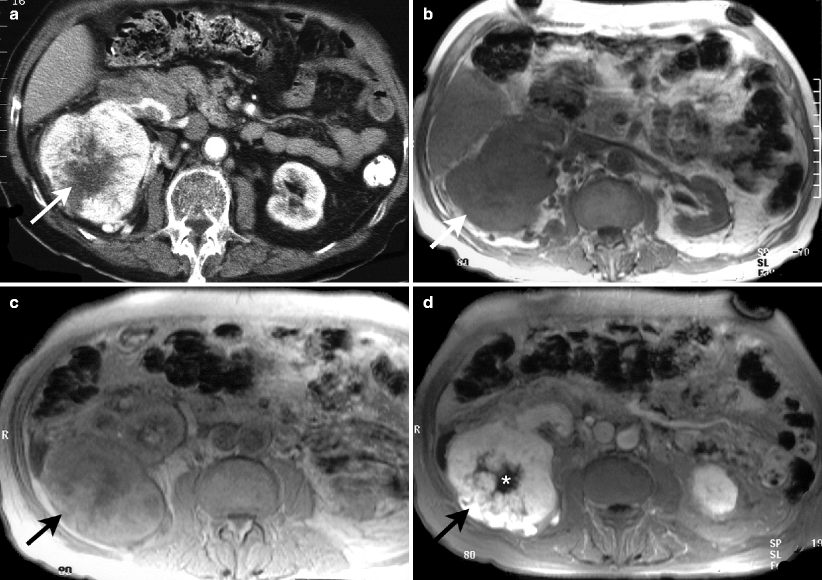
Fig. 1.2
CT and MRI findings of oncocytoma. (a) Axial CT image at nephrogram phase demonstrates avidly enhancing large mass arising from right kidney with stellate shape central scar tissue (arrow) which shows no enhancement. (b) Axial T1-weighted MRI of same patient reveals hypointense right kidney mass (arrow). (c) The lesion has intermediate signal intensity on proton density image (arrow). (d) Axial fat-saturated T1-weighted image after intravenous gadolinium administration reveals contrast enhancement of mass (arrow). Unenhanced central region of the lesion represents scar (*)
CT cannot distinguish between an oncocytoma and RCC.
Magnetic Resonance Imaging
Oncocytoma is isointense to hypointense to the normal renal parenchyma on T1-weighted images and has a variable appearance on T2-weighted images (Fig. 1.2b, c).
Central scar as in CT suggests the diagnosis of oncocytoma (Fig. 1.2d).
Postgadolinium enhancement of oncocytoma is less than the surrounding normal renal parenchyma.
Angiography
Spoke-wheel pattern, homogenous nephrogram, and a smooth margin
Pathology
Oncocytoma is derived from the intercalated cells of renal collecting tubules and accounts for about 5 % of surgically excised renal neoplasms (Figs. 1.3 and 1.4).
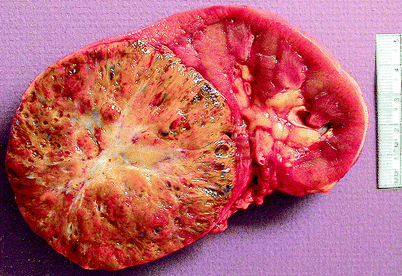
Fig. 1.3
Oncocytoma. Renal oncocytomas are typically solid and mahogany brown, less often tan to pale yellow and well circumscribed, with varying degrees of encapsulation. They range from 0.3 to 26 cm in greatest dimension. About one-third show central scarring; infrequently, they exhibit hemorrhage, focal cystic degeneration, tumor extension into perirenal fat, and, rarely, tumor growth into large blood vessels
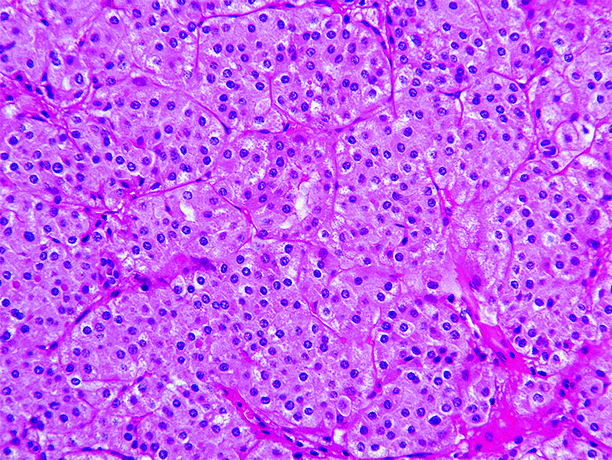
Fig. 1.4
Oncocytoma. Oncocytoma is composed mainly of round to polygonal cells with densely granular eosinophilic cytoplasm, round uniform nuclei with smoothly distributed chromatin, and a central nucleolus. Mitotic figures are absent or rare. Findings that exclude the diagnosis of oncocytoma include areas of clear cell or spindle cell carcinoma, prominent papillary architecture, macroscopic or conspicuous microscopic necrosis, significant numbers of mitotic figures, and atypical mitotic figures
Males are affected about twice as often as females, and average patient age is 62 years. Rare cases in children have been reported.
Although oncocytoma is usually asymptomatic, and most are discovered incidentally, it presents occasionally with the “classic triad” of hematuria, flank pain, and palpable abdominal mass.
Most oncocytomas are solitary, but rare cases of multiple oncocytomas arising in a single kidney (renal oncocytomatosis) occur, and in 4 % of cases, oncocytoma is bilateral. It is considered benign.
Papillary Adenoma
Papillary adenomas are 5 mm or less in diameter in size and typically subcapsular in location.
They are very common, being found in 21 % of patients overall, in 10 % of patients aged 21–40 years, and in 40 % of patients aged 70–90 years, and they are particularly common in patients undergoing chronic dialysis, being present in 33 % of patients with acquired cystic renal disease.
Since by definition they are 5 mm or less in diameter, they are not detectable by radiological studies.
Metanephric Neoplasms
Metanephric neoplasms are a heterogeneous group of renal neoplasms that include metanephric adenoma (epithelial tumor), metanephric stromal tumor (stromal neoplasm), and metanephric adenofibroma (mixed epithelial and stromal neoplasm), all of which are considered benign. A single well-documented case of metanephric adenosarcoma has been reported.
Metanephric Adenoma
General Information
Metanephric adenomas account for 0.2 % of adult renal epithelial tumors.
Metanephric adenoma is asymptomatic in approximately 50 % of patients; abdominal pain and hematuria are common clinical symptoms.
Polycythemia, a characteristic finding seen in approximately 10 % of patients with metanephric adenoma, completely resolves after surgical resection.
Imaging
Ultrasound
Their appearance on sonography is variable. Exophytic solid lesion with irregular contours and cystic areas within the lesion may be observed.
Computed Tomography
Well-demarcated and round lesions.
These tumors are usually solitary and hyperdense on unenhanced CT and are hypovascular on enhanced CT.
Calcification is seen in 20 % of tumors.
Magnetic Resonance Imaging
These tumors are hypo- or isointense on T1-weighted and slightly hyperintense on T2-weighted MRI sequences.
Pathology
Metanephric adenoma is the commonest of a class of rare renal tumors that also include metanephric stromal tumor, metanephric adenofibroma, and metanephric adenosarcoma (Figs. 1.5 and 1.6).
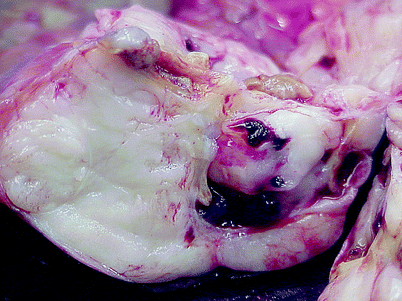
Fig. 1.5
Metanephric adenoma. Metanephric adenoma ranges from 0.3 to 20 cm in diameter. It is typically unilateral and unifocal and is either unencapsulated or invested with only a limited and discontinuous pseudocapsule. Tumors are tan to gray to yellow, soft to firm, and most are solid, but some have areas of hemorrhage, necrosis, and cystic degeneration, as exemplified in this image. Calcifications are often present in solid areas or in the walls of cystic structures
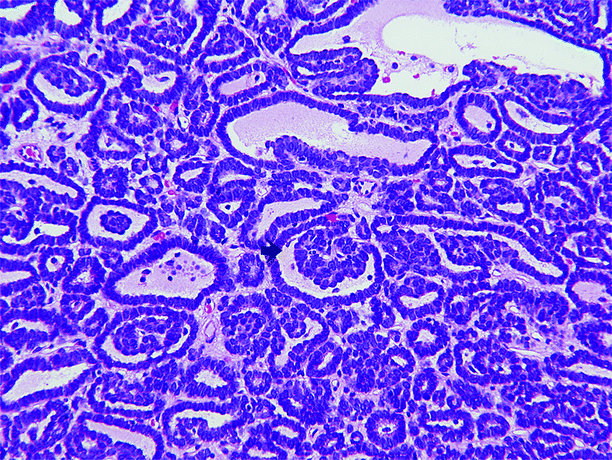
Fig. 1.6
Metanephric adenoma. Metanephric adenoma is composed of very small acini separated by variable amounts of acellular edematous or hyalinized stroma. Tumor cells are closely spaced, often overlapping, and have minimal cytoplasm. Their nuclei are small and dark and lack nucleoli; mitotic figures are absent or rare. About half of cases show papillary structures consisting of polypoid fronds or short papillary infoldings within tubular or cystic spaces, producing a glomeruloid appearance (arrow)
It occurs in children and adults and is twice as common in females as in males.
It is usually asymptomatic, although some patients have been noted to have polycythemia; most cases have been discovered incidentally. Its biologic behavior is benign.
Metanephric Stromal Tumor
General Information
Rare benign stromal tumor of the kidney. Most are diagnosed in the first decade of life. Presenting symptom is usually abdominal mass.
Imaging
Ultrasound
Heterogeneous mass with solid and cystic components. Solid component is isoechoic to renal parenchyma.
Computed Tomography
Hypodense mass on unenhanced CT with lobulated contours.
Contrast-enhanced CT reveals slight peripheral nodular contrast enhancement on delayed venous phase.
Magnetic Resonance Imaging
Lesions show low signal intensity on T1-weighted images. Only cystic portions can be visualized separately from renal parenchyma on T2-weighted images.
Contrast enhancement pattern is similar to CT.
Metanephric Adenofibroma
Occurs in children and young adults.
Comprised of a mixture of stromal elements (identical to those in metanephric stromal tumor) and well-defined areas of immature epithelium (tubules and papillae).
Mesenchymal Neoplasms
Angiomyolipoma (AML)
General Information
AMLs are benign neoplasms composed of blood vessels, smooth muscle, and fatty tissue.
May be sporadic as well as associated with tuberous sclerosis. Sporadic occurrence is much more common, accounting for 80–90 % of cases of AML (Fig. 1.7a–c).
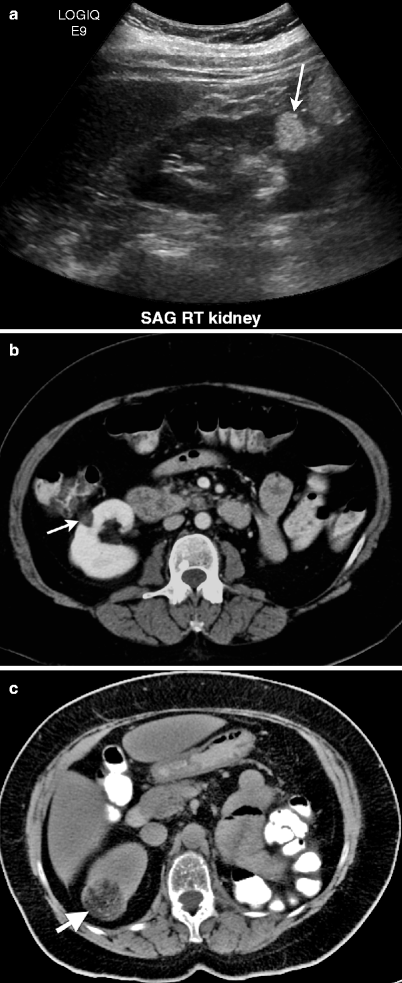
Fig. 1.7
Angiomyolipoma. (a) Longitudinal view of gray scale ultrasound demonstrates hyperechoic mass (arrow) in the renal parenchyma of lower third of the right kidney. (b) Axial contrast-enhanced CT of same patient reveals well-defined fat-containing mass (arrow) with minimal enhancement arising from right kidney. (c) Unenhanced CT of another patient demonstrates a fat-containing mass (arrow) in the right kidney
Up to 80 % of patients with tuberous sclerosis have AMLs, which are usually multiple and bilateral (Fig. 1.8a–d). In tuberous sclerosis, tumors of various types arise in the brain, retina, kidneys, heart, and skin (Fig. 1.8e, f).
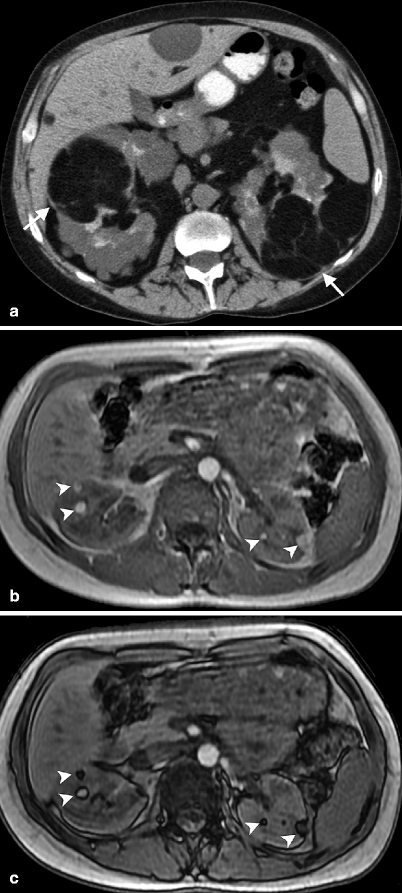
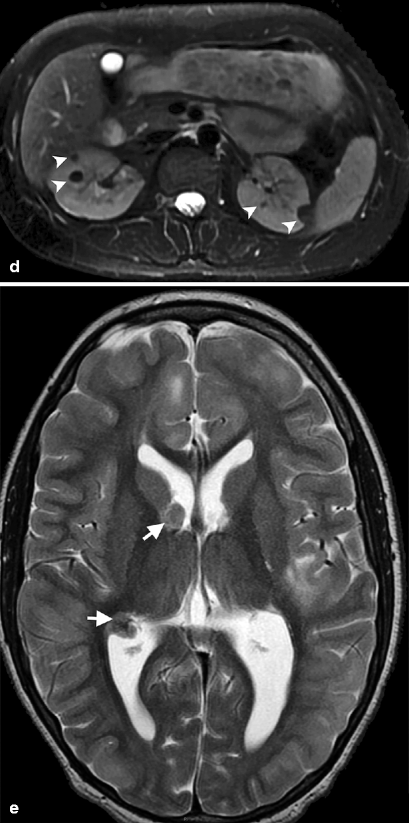
Fig. 1.8
Bilateral angiomyolipomas in tuberous sclerosis. (a) Noncontrast-enhanced CT demonstrates multiple, lobulated, hypodense fat-containing bilateral renal masses (arrows). (b) Bilateral renal angiomyolipomas (arrowheads) in a patient with tuberous sclerosis appear hyperintense on in-phase image of dual-echo sequence. (c) Out-of-phase image reveals signal loss around the lesions (arrowheads) which confirms fat content of AMLs. (d) Bilateral AMLs present with signal loss and appear hypointense (arrowheads) on axial fat-saturated T2-weighted MRI. (e) Axial T2-weighted MRI of another patient with tuberous sclerosis demonstrates subependymal tubers (arrows)
Larger tumors may be symptomatic presenting with pain, hematuria, and intratumoral hemorrhage.
Imaging
Ultrasound
Round or oval well-circumscribed tumors. These tumors may be exophytic or intracortical.
AMLs are identified on ultrasound by the intense echogenic appearance of the fat within these tumors (Fig. 1.7a).
Although highly suggestive, echogenicity is not pathognomonic of AMLs and may also be rarely seen in RCCs.
Sometimes they may appear heterogeneous secondary to hemorrhage and necrosis (Fig. 1.9). They have high propensity to hemorrhage when larger than 4 cm in size.
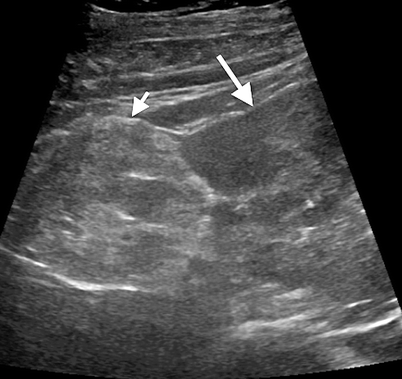
Fig. 1.9
Angiomyolipoma. Gray-scale ultrasound demonstrates an exophytic angiomyolipoma (short arrow) with a heterogeneous appearance arising from the kidney (long arrow)
Computed Tomography
Well-circumscribed renal masses with intratumoral macroscopic fat.
Lesions without detectable fat cannot be distinguished from other renal neoplasms on CT. Approximately 5 % of AMLs do not show fat attenuation on CT scans and cannot be differentiated from renal cell cancer (Fig. 1.7b). Fat may also be obscured secondary to intratumoral hemorrhage.
Although AMLs are benign tumors, they may show evidence of extension into the renal vein and inferior vena cava.
Bleeding angiomyolipomas have heterogeneous appearance on CT with blood density (Fig. 1.10).
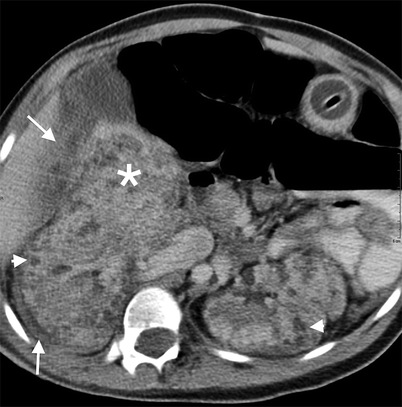
Fig. 1.10
AML with hemorrhage. Contrast-enhanced CT reveals bilateral hypodense AMLs (arrowheads). A heterogeneous hematoma (*) medial to the kidney is visualized with increased perirenal density (arrows)
Magnetic Resonance Imaging
AML is identified on opposed phase chemical shift MRI by the presence of India ink artifact (loss of signal at the interface of fat- and nonfat-containing areas) at the interface of mass and renal parenchyma, or within the renal mass (Fig. 1.8c).
Fat-saturated T1- and T2-weighted sequences can confirm the presence of fat by demonstrating loss of signal (Fig. 1.8d).
Pathology
The majority of patients with AML are female (Figs. 1.11 and 1.12).
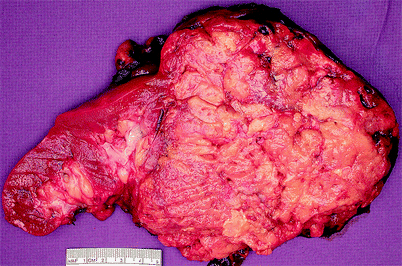
Fig. 1.11
Angiomyolipoma (AML). Angiomyolipomas may be located in the renal cortex, medulla, or capsule and may be solitary or multiple. Most that are removed surgically are greater than 4 cm in diameter and can be as large as 30 cm in greatest dimension. They are typically smoothly rounded or ovoid, circumscribed but not encapsulated. They may compress and distort adjacent normal kidney but do not infiltrate it. Infrequently, extension into perirenal fat or renal vein is observed. A tumor composed mainly of fat may resemble lipoma, and one mainly composed of smooth muscle may mimic leiomyoma
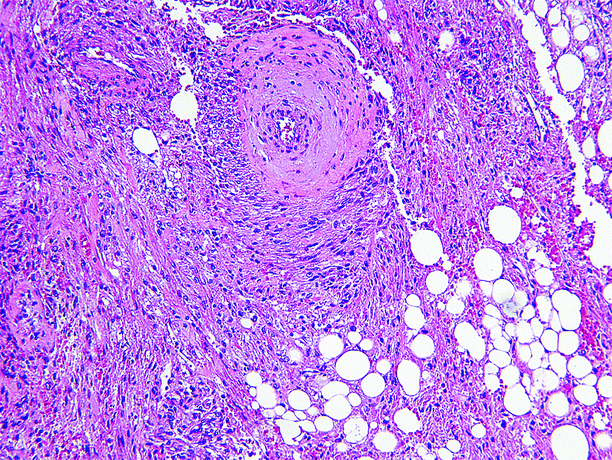
Fig. 1.12
Angiomyolipoma (AML). The microscopic appearance of AML varies according to the proportions of blood vessels, muscle, and fat comprising the tumor. Smooth muscle may be arranged in strands or fascicles or radially arranged at the periphery of large blood vessels, producing a “hair-on-end” appearance. Blood vessels are usually thick walled, and their lumens may be eccentric or very small. Fat cells exhibit their usual morphology
AML is a true neoplasm, about half of which arise sporadically; the other half occur in patients with tuberous sclerosis, a heritable genetic disorder linked to loss of heterozygosity on chromosome 16p.
Up to 80 % of patients with tuberous sclerosis develop AML, most often between the ages of 25–35 years, and AMLs arising in this setting tend to be small, multiple, bilateral, and asymptomatic. In contrast, most patients who develop sporadic AML are between 45 and 55 years old at the time of diagnosis, and their tumors are more often large and solitary and more likely to cause symptoms (such as flank pain) than heritable AML.
Although it is intrinsically benign, angiomyolipoma has been associated with a variety of complications, the most common of which is hemorrhage, particularly in tumors larger than 4 cm. Less common complications include unresectability due to infiltration of local structures, the development of secondary leiomyosarcoma, and obliteration of functioning renal parenchyma by innumerable bilateral tumors and subsequent renal insufficiency in patients with tuberous sclerosis.
Juxtaglomerular Neoplasm (Reninoma)
General Information
Rare tumors, usually presenting with hypertension and hypokalemia due to hypersecretion of renin hormone.
Most commonly occurs in young women in the reproductive age group.
Imaging
Ultrasound
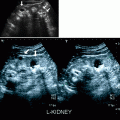
Smooth well-marginated homogeneous mass, usually hypo- to isoechoic (Fig. 1.13a). Lesion is located within the renal cortex.
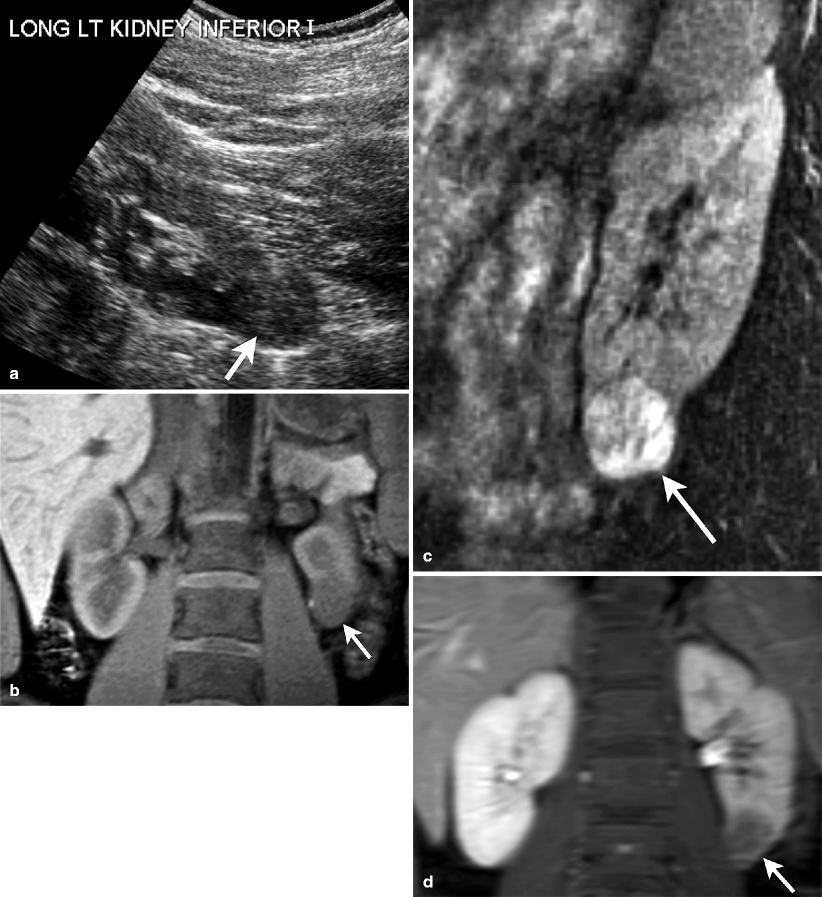
Fig. 1.13
Juxtaglomerular neoplasm (Reninoma). (a) Gray-scale ultrasound demonstrates a hypoechoic exophytic solid mass (arrow) arising from inferior portion of the left kidney. (b) Unenhanced coronal T1-weighted MRI reveals well-demarcated hypointense mass (arrow). (c) Lesion (arrow) enhances on contrast-enhanced coronal T1-weighted image. (d) Lesion exhibits washout and appears hypointense (arrow) on delayed phase contrast-enhanced T1-weighted image
Computed Tomography
Smooth and sharply marginated mass.
May appear heterogeneous in the presence of hemorrhage.
Minimal enhancement on contrast-enhanced CT.
Pathology
Juxtaglomerular cell tumor arises from specialized smooth muscle cells that comprise the vasculature of the juxtaglomerular apparatus (Figs. 1.14 and 1.15).
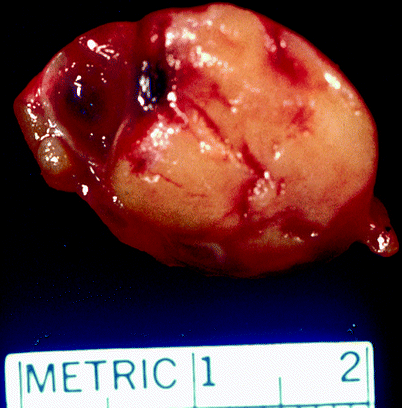
Fig. 1.14
Juxtaglomerular cell tumor. Juxtaglomerular cell tumors are usually well circumscribed, with a fibrous capsule of variable thickness. They are typically 2–4 cm in diameter, but can be much larger. Their cut surfaces are yellow to gray/tan and often hemorrhagic
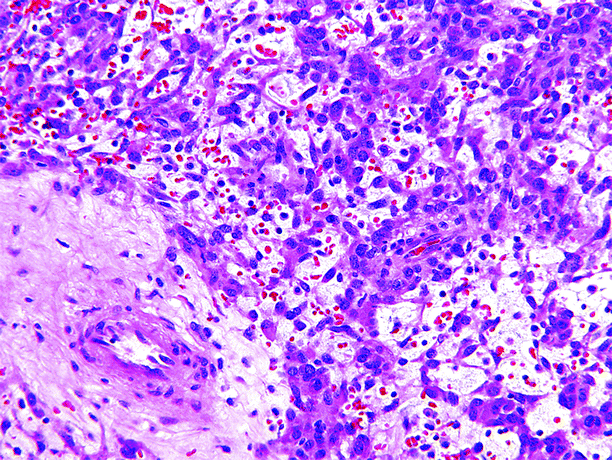
Fig. 1.15
Juxtaglomerular cell tumor. Juxtaglomerular cell tumors are composed of polygonal, round, or elongated spindle-shaped cells with slightly eosinophilic cytoplasm and centrally located nuclei, set in a scant edematous stroma. Most tumors have scattered thick-walled blood vessels (lower left) and a scant infiltrate of inflammatory cells. Mitotic figures are usually absent
It is twice as more common in females than in males. Although patient age range is from 6 to 69 years, the majority of patients are in their 20s and 30s, with a mean age of 27 years. Clinical findings are distinctive and often diagnostic preoperatively. Patients report pain, headache, polyuria, nocturia, dizziness, and vomiting, and almost all have labile and refractory hypertension. They typically have high serum renin levels, elevated serum aldosterone, and hypokalemia. Surgical excision of the tumor usually normalizes the patient’s blood pressure and relieves other symptoms.
With a single well-documented exception, these tumors are usually not complicated by recurrence or metastasis postoperatively.
Leiomyoma
General Information
Leiomyomas are rare benign spindle cell tumors that are found in approximately 5 % of autopsy specimens.
They may originate from smooth muscles in the renal capsule, pelvis, calyx, or blood vessels.
These tumors are usually small, although the few clinically apparent tumors may be larger.
Imaging
Ultrasound
Renal leiomyomas present as solid lesion on ultrasound.
Cystic changes are uncommon.
Computed Tomography
Well-circumscribed, solid, peripheral lesions, the majority of which are capsular or subcapsular in location (Fig. 1.16).
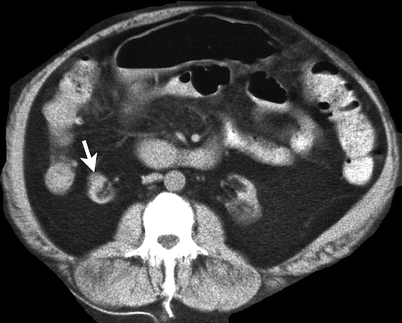
Fig. 1.16
Renal leiomyoma. Unenhanced CT demonstrates a round hypodense mass (arrow) in the anterior portion of the atrophied right kidney
There may be a cleavage plane between the leiomyoma and the renal cortex, or the lesion may be totally exophytic or attached to the cortex by only a small stalk.
Cannot be differentiated from a renal leiomyosarcoma or RCC based on imaging findings alone. The diagnosis of leiomyoma is excluded in lesions that exhibit invasion or metastasis.
Magnetic Resonance Imaging
Heterogeneous signal intensity on T1- and T2-weighted images
Internal areas of hypointensity on T1-weighted images
Pathology
The largest reported renal leiomyoma was 57.5 cm in diameter and weighed 37 kg. The average size of renal leiomyomas is about 12 cm.
Similar to leiomyomas in other sites, they are typically firm, bulging, and well circumscribed, with a white whorled fibrous or trabeculated cut surface (Fig. 1.17).
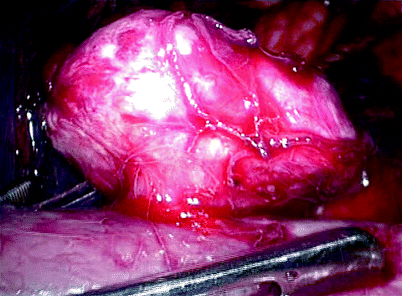
Fig. 1.17
Renal leiomyoma. This image is an intraoperative photo of a renal capsular leiomyoma removed laparoscopically (From MacLennan GT, Cheng L. Neoplasms of the kidney. In: Bostwick DG, Cheng L, editors. Urologic Surgical Pathology. 2nd ed. Edinburgh: Mosby/Elsevier; 2008, with permission)
Microscopically, they are composed of spindled cells, usually arranged in small intersecting fascicles. Nuclear pleomorphism is minimal, and necrosis and mitotic figures are absent. Adipocytes and abnormal blood vessels are absent.
Capsular leiomyomas often contain populations of cells strongly immunopositive for HMB-45, suggesting an undefined relationship with angiomyolipoma (Fig. 1.18).
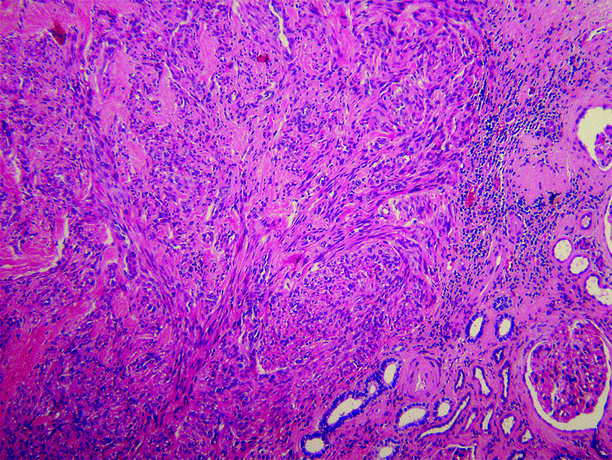
Fig. 1.18
Renal leiomyoma. Lesion is composed of spindle cells arranged in intersecting fascicles. Normal renal parenchyma is at lower right
Lymphangioma
General Information
Lymphangiomas are rare benign lesions of vascular origin that show lymphatic differentiation.
They are typically located in the renal hilum or in the perinephric space.
Diffuse renal lymphangiomatosis may occur.
Imaging
Ultrasound
Cystic masses with anechoic appearance and septa formations.
Renal lymphangiomas may manifest with solid appearance due to the reverberations that occur between microscopic lymph spaces and surrounding connective tissue.
Computed Tomography
Most renal lymphangiomas are focal lesions and may appear as unilocular or multilocular hypodense cysts on CT (Fig. 1.19).
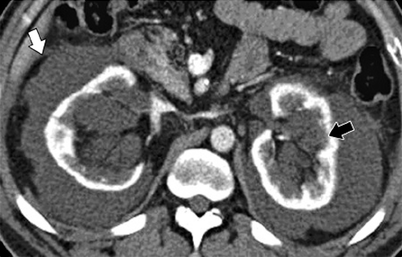
Fig. 1.19
Renal lymphangioma. Contrast-enhanced CT image shows bilateral multiple cystic masses in the perinephric (white arrow) and renal sinus (black arrow) regions, consistent with lymphangiomas. (With permission from Katabathina VS, Vikram R, Nagar AM, Tamboli P, Menias CO, Prasad SR. Mesenchymal neoplasms of the kidney in adults: imaging spectrum with radiologic-pathologic correlation. Radiographics. 2010;30(6):1525–40)
The CT appearance of renal lymphangiomas can vary according to their fluid content, which may be mucoid or hemorrhagic in nature.
Magnetic Resonance Imaging
They usually appear hypointense on T1-weighted and hyperintense on T2-weighted images (Fig. 1.20). Hemorrhage or increased protein content can increase signal intensity of lesion on T1-weighted images.
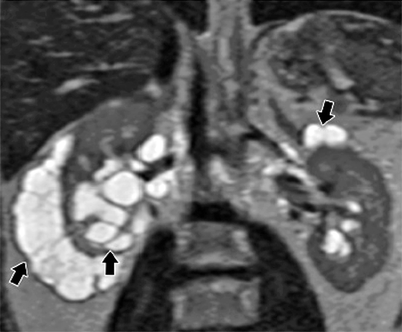
Fig. 1.20
Lymphangioma in a 45-year-old man. Coronal T2-weighted MR image shows bilateral multilocular cystic masses (arrows) in the perinephric and renal sinus regions, consistent with lymphangiomas. (With permission from Katabathina VS, Vikram R, Nagar AM, Tamboli P, Menias CO, Prasad SR. Mesenchymal neoplasms of the kidney in adults: imaging spectrum with radiologic-pathologic correlation. Radiographics. 2010;30(6):1525–40)
Pathology
Renal lymphangioma is typically a well-encapsulated multicystic mass that may be unilateral or bilateral, localized or diffuse. The cut surface is composed of innumerable fluid-filled cysts ranging from 0.1 to 2.0 cm in diameter, often mimicking a polycystic kidney disease or a multilocular renal cyst (Fig. 1.21a).
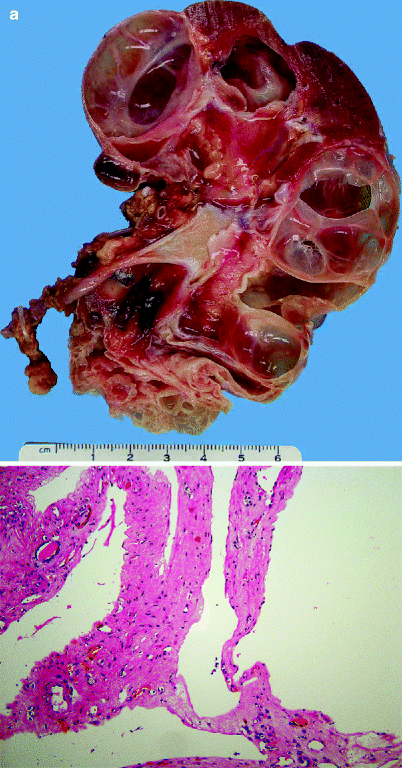
Fig. 1.21
Lymphangioma. (a) Lesion is a well-encapsulated multicystic mass. Its cut surface is composed of innumerable fluid-filled cysts. (b) Lymphangioma. Cystic spaces are separated by delicate fibrous septa lined by flattened endothelial-type cells
Microscopically, lymphangioma consists of numerous thin-walled cysts separated by delicate fibrous septa. The cysts are lined by flattened endothelial cells and are separated by septal structures that may contain normal renal structures such as glomeruli, tubules, and blood vessels (Fig. 1.21b).
Hemangioma
General Information
Uncommon benign renal tumor.
Most frequent location is the tip of the papilla.
Renal hemangiomas are bilateral in 12 % of cases.
Renal hemangiomas may present with episodes of hematuria and renal colic.
Imaging
Ultrasound
They have variable echotexture on sonography.
Sonographic appearance of renal hemangiomas is usually similar to liver hemangiomas.
Computed Tomography
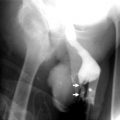
CT angiography reveals enhancement of thick vascular channels and dilated renal vein (Fig. 1.22a–c).
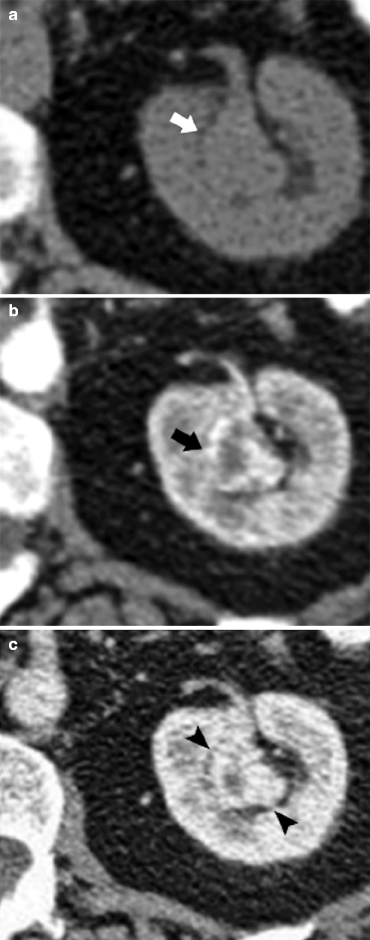
Fig. 1.22
Renal hemangioma in a 25-year-old woman. (a) Nonenhanced CT image shows a lobulated isoattenuating soft tissue mass (arrow) in the region of the renal sinus. (b) Axial contrast-enhanced CT image obtained during the arterial phase shows intense enhancement (arrow) of the lesion. (c) Axial contrast-enhanced CT image obtained during the portal venous phase shows persistent enhancement (arrowheads) of the lesion. At histopathologic examination, this lesion was determined to be a capillary hemangioma (With permission from Katabathina VS, Vikram R, Nagar AM, Tamboli P, Menias CO, Prasad SR. Mesenchymal neoplasms of the kidney in adults: imaging spectrum with radiologic-pathologic correlation. Radiographics. 2010;30(6):1525–40)
Magnetic Resonance Imaging
Like hemangiomas in other regions of the body, they are hyperintense on T2-weighted images.
Flow voids may be seen as punctate signal loss on T2-weighted images.
Persistent contrast enhancement on delayed images is fairly characteristic of renal hemangiomas.
Pathology
Although most renal hemangiomas are only 1–2 cm in greatest dimension, they may be as large as 18 cm (Fig. 1.23a).
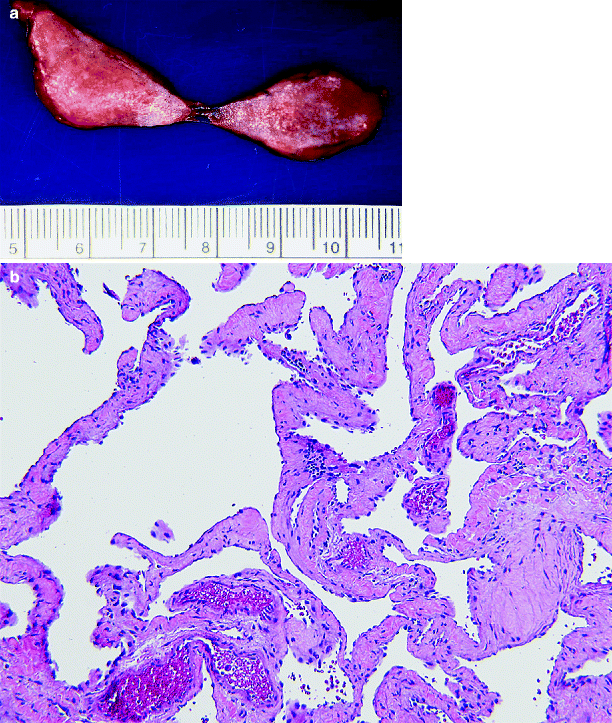
Fig. 1.23
Hemangioma. (a) On CT scan, this was a solid mass lesion in the renal hilum. At surgery, it was peeled off the renal vein. The lesion is red and has a slightly spongy cut surface (From MacLennan GT, Cheng L. Neoplasms of the kidney. In: Bostwick DG, Cheng L, editors. Urologic Surgical Pathology. 2nd ed. Edinburgh: Mosby/Elsevier; 2008, with permission) (b) Hemangioma. Irregular blood-filled vascular spaces lined by a single layer of endothelial cells that lack mitotic activity and nuclear pleomorphism. The lesion shown here is a cavernous hemangioma; capillary hemangiomas have also been reported
The commonest locations are the renal pelvis or renal pyramids, but hemangiomas also arise in the renal cortex, the renal capsule, or within peripelvic blood vessels or soft tissues. Those that involve the renal pelvis or a papilla may be very hard to identify grossly, appearing as a small mulberry-like lesion or a small red streak. Larger lesions often appear red or gray tan and spongy.
Microscopically, hemangiomas consist of irregular blood-filled vascular spaces lined by a single layer of endothelial cells that lack mitotic activity and nuclear pleomorphism. They are typically either of cavernous or capillary type (Fig. 1.23b).
Renomedullary Interstitial Cell Tumor (Medullary Fibroma)
Renal fibroma is found in the renal medulla. It arises from the interstitial cells of the renal medulla.
These tumors are benign and rarely clinically significant. Most are discovered incidentally, although rarely, they are large enough to obstruct renal pelvic outflow and cause pain.
They usually appear as solitary and well-defined nodules.
On CT, fibromas appear as lobulated hypodense masses.
Renal fibromas have an intermediate signal intensity on T1-weighted images and isointense or hyperintense relative to normal renal parenchyma on T2-weighted images.
Pathology
Tumors are gray white. Usual tumor size is less than 0.3 cm; however, tumors >6 cm in diameter have been reported (Fig. 1.24a).
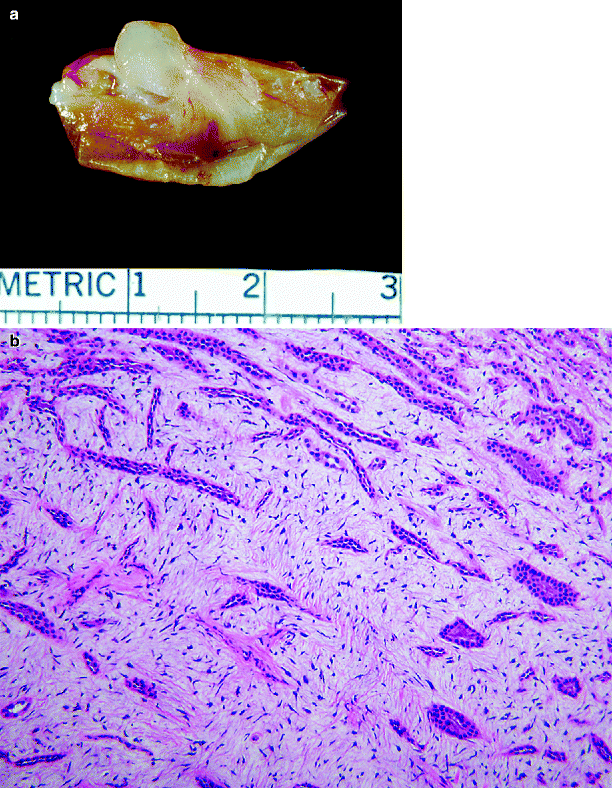
Fig. 1.24
Renomedullary interstitial cell tumor. (a) A small gray-white tumor involves the tip of a papilla (From MacLennan GT, Resnick MI, Bostwick DG. Pathology for Urologists. Philadelphia: Saunders; 2003, with permission). (b) Renomedullary interstitial cell tumor. Collecting ducts are surrounded by spindled and stellate cells in a loose basophilic stroma
The tumor is composed of small stellate or spindled cells set in a background of loose faintly basophilic stroma containing interlacing bundles of delicate fibers and typically infiltrating the interstitium between collecting ducts (Fig. 1.24b).
Schwannoma
Renal schwannoma is rare. A schwannoma may arise from renal parenchyma, renal hilar soft tissues, or renal capsule.
Ultrasound reveals a well-defined, hypoechoic mass.
On CT, schwannoma presents as a solitary well-circumscribed, rounded, or lobulated hypodense enhancing lesions.
Pathology
Schwannomas range from 4 to 16 cm in size. They are typically well circumscribed, tan to yellow, sometimes multinodular, and have a dense fibrous capsule (Fig. 1.25a).
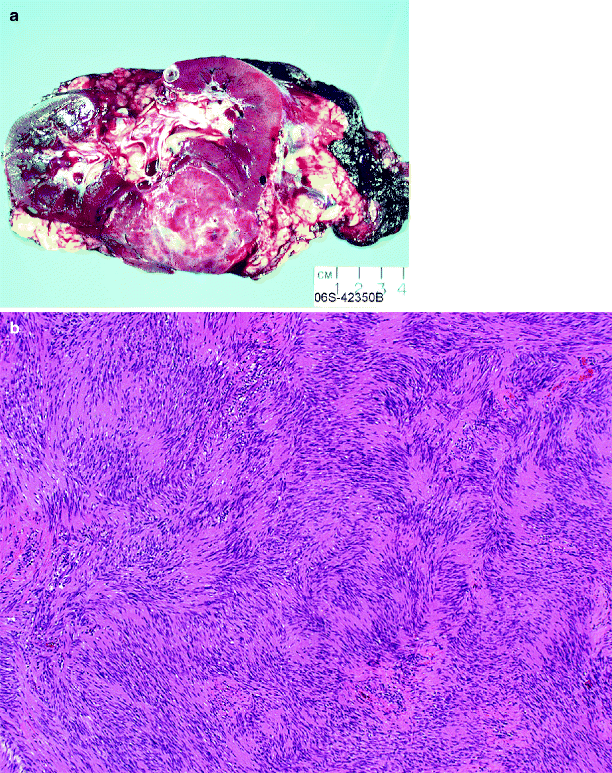
Fig. 1.25
Renal schwannoma. (a) A well-circumscribed solid tumor in a young female (From MacLennan GT, Cheng L. Neoplasms of the kidney. In: Bostwick DG, Cheng L, editors. Urologic Surgical Pathology. 2nd ed. Edinburgh: Mosby/Elsevier; 2008, with permission) (b) Renal schwannoma. Antoni A areas consist of compact spindle cells with twisted nuclei, with characteristic palisading
Schwannomas are classically composed of compact spindle cells with twisted nuclei. Approximately parallel rows of well-aligned palisaded tumor cells are separated by fibrillary cell processes, a formation designated as a Verocay body. At low power, the cells are often arranged as areas of dense cellularity (Antoni A area) alternating with paucicellular (Antoni B) areas (Fig. 1.25b). The proportions of these elements vary, and the areas may subtly blend into one another. Prominent blood vessels may be present. Mitotic figures are absent or rare, and necrosis is absent. Tumor cells show diffuse strongly positive immunostaining for S-100 protein.
Congenital Mesoblastic Nephroma
General Information
Most common solid renal tumor in the newborn period.
Presentation is usually a palpable abdominal mass in young infants.
Imaging
Ultrasound
Homogeneous well-defined solid renal mass.
It may appear heterogeneous in the setting of hemorrhage or necrosis.
Computed Tomography
CT may be helpful to demonstrate the calcification and fat within the mass.
Magnetic Resonance Imaging
Mesoblastic nephroma manifests on MRI with low signal intensity on T1-weighted and high signal intensity on T2-weighted images.
Pathology
Mesoblastic nephroma is the most common renal neoplasm in patients less than 3 months old. It accounts for 2–4 % of pediatric renal neoplasms (Figs. 1.26 and 1.27).
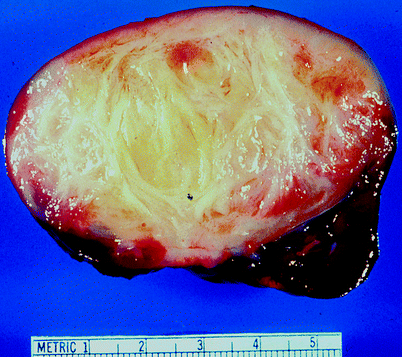
Fig. 1.26
Congenital mesoblastic nephroma (CMN). Mesoblastic nephroma presents in either classic form (24 %), cellular form (66 %), or as a mixture of these forms (about 10 %). Classic CMN, shown in this image, has a firm, whorled appearance and an indistinct interface with surrounding normal kidney. Cellular CMN has a more sharply circumscribed outline; it usually appears softer with cystic and hemorrhagic areas (From MacLennan GT, Resnick MI, Bostwick DG. Pathology for Urologists. Philadelphia: Saunders; 2003, with permission)
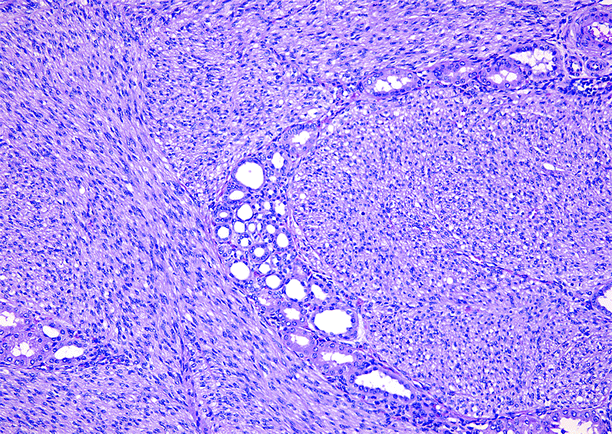
Fig. 1.27
Congenital mesoblastic nephroma (CMN). In classic CMN, shown here, nuclei are elongated, bland, and uniform, and mitotic figures are infrequent. Tumor cells infiltrate around tubules and glomeruli at the interface between tumor and normal parenchyma. Cellular CMN is more densely cellular, less infiltrative at the interface between tumor and normal kidney, and much more mitotically active. Prognosis is dependent upon patient age and completeness of surgical excision, rather than on tumor morphology
Almost all reported cases have occurred in individuals less than 30 months old; 90 % of cases occur during the first 12 months of life.
The commonest mode of discovery is by incidental palpation of an abdominal mass in an infant.
It presents in two forms, classic and cellular. Recurrence is rare and is attributed to incomplete resection. Rarely, death related to metastasis is reported.
Mixed Epithelial and Mesenchymal Tumors
Cystic Nephroma
General Information
Cystic nephroma is a benign renal neoplasm composed entirely of epithelial-lined cysts separated by septa of variable thickness.
Historically, it has been a matter of controversy concerning the definition of what constitutes the term “cystic nephroma” and the relationship between cystic nephroma and cystic partially differentiated nephroblastoma.
Currently, the diagnosis of cystic nephroma is restricted to adults; renal neoplasms in children composed entirely of epithelial-lined cysts separated by septa of variable thickness and lacking expansile nodules to alter the rounded contour of the cysts are diagnosed as cystic partially differentiated nephroblastoma, regardless of the presence or absence of blastema or other immature elements in the septa.
Cystic nephroma in adults is seen mainly in females (8:1 female:male ratio), is rare before age 30, and does not exhibit renal nephrogenic rests or skeletal muscle fibers in the septa. Conversely, cystic partially differentiated nephroblastoma is slightly more common in males, is rare after the age of 2 years, sometimes exhibits renal nephrogenic rests, and often exhibits skeletal muscle fibers in the septa.
In summary, it is currently accepted that the traditional term “cystic nephroma” embraces two entirely different lesions, one of which (cystic nephroma) predominantly occurs in adult females and has no relationship to Wilms’ tumor, and the other (cystic partially differentiated nephroblastoma), a lesion of young children of both genders, probably represents a mature form of Wilms’ tumor.
Cystic nephroma may be detected as a painless abdominal mass or may cause flank or abdominal pain or hematuria. Most are discovered incidentally. Patients range in age from 22 to 79 years old with a mean age of about 55–60 years, and the great majority are female.
Cystic nephroma is considered to be a benign neoplasm, cured by adequate surgical excision, and recurrence is usually attributed to incomplete resection. Nonetheless, there are a few well-documented instances of sarcoma arising in a background of cystic nephroma in adults, sometimes with a fatal outcome.
Imaging
Ultrasound
Multicystic mass with thin echogenic septa, without any solid or nodular elements (Fig. 1.28a).
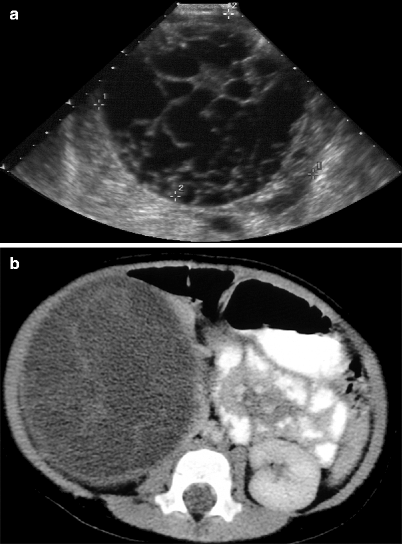
Fig. 1.28
Multilocular cystic renal tumor. (a) Gray-scale ultrasound demonstrates multiloculated cystic mass with thick septa formations and well-defined contour. (b) Axial contrast-enhanced CT reveals a well-defined, hypodense cystic mass with septa formations
A claw or beak shape of adjacent normal renal parenchyma can help confirm the renal origin of the mass.
May be associated with urinary tract obstruction if herniation into the renal pelvis is present.
Computed Tomography
Well-encapsulated multilocular mass separated by thin septa which may show enhancement (Figs. 1.28b and 1.29).
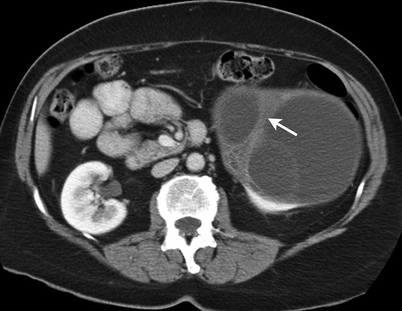
Fig. 1.29
Multilocular cystic renal tumor. Contrast-enhanced CT demonstrates a multiloculated cystic mass with enhancing thick septa (arrow)
The tumor often herniates into the renal pelvis causing a filling defect and sometimes also causes obstruction.
Magnetic Resonance Imaging
Similar findings as on CT with variable appearance of the cysts on T1- and T2-weighted sequences depending on the cyst contents
Pathology
Cystic nephroma is typically well circumscribed, usually unilateral but rarely bilateral, and ranges in size from 1.4 to 13 cm, with an average diameter of approximately 6 cm. Most tumors involve the renal cortex, but rarely may be predominantly intrapelvic.
Cystic nephroma is composed entirely of epithelial-lined cysts separated by septa of variable thickness (Figs. 1.30 and 1.31). The lining epithelium is flattened, cuboidal, or hobnail in appearance and lacks mitotic activity. The septal stroma of the septa is composed of spindle cells in a collagenous background. In some areas, stromal cell nuclei are very closely packed, imparting an appearance reminiscent of ovarian stroma.
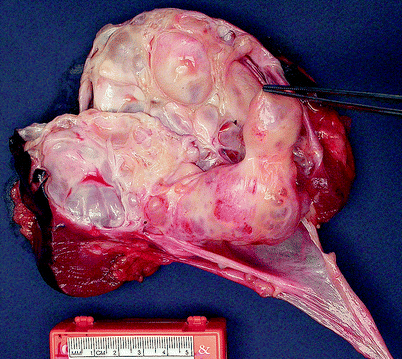
Fig. 1.30
Cystic nephroma. Cystic nephromas are sharply demarcated, usually unilateral but rarely bilateral, and range in size from 1.4 to 13 cm, with an average diameter of approximately 6 cm. Most tumors are limited to the renal cortex, but occasionally they protrude into the renal pelvis, as shown in this image (Image courtesy of Carmen Frias-Kletecka, M.D.)
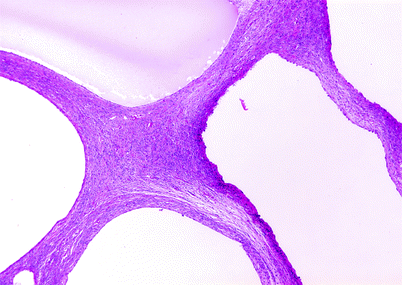
Fig. 1.31
Cystic nephroma. Cysts are enclosed by septa of variable thickness, but by definition less than 5 mm; expansile nodules are absent. The septal stroma is composed of spindle cells in a collagenous background. Cysts are lined by flattened, cuboidal, or hobnail-type epithelial cells; some lining cells may possess clear cytoplasm. Mitotic figures are absent
Mixed Epithelial and Stromal Tumor of Kidney (MESTK)
This is a recently recognized distinct neoplasm. These tumors are relatively rare with a female preponderance. Imaging studies are not diagnostic but reveal a solid or solid and cystic mass in most cases (Fig. 1.32).
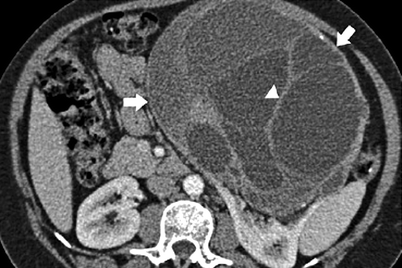
Fig. 1.32
MEST. CT image acquired during the corticomedullary phase of enhancement shows low-level enhancement of the wall (arrows) and septations (arrowhead) of this complex cystic mass (With permission from Prasad SR, Dalrymple NC, Surabhi VR. Cross-sectional imaging evaluation of renal masses. Radiol Clin North Am. 2008;46(1):95–111, vi–vii)
The clinical course after resection of MESTK is typically benign, but there have been reports of cases with clear-cut sarcomatoid morphology, aggressive clinical behavior, and ultimately fatal outcomes.
Pathology
This is a renal neoplasm that is macroscopically solid and cystic and microscopically biphasic, composed of glands, often cystically dilated, and solid areas of spindle cells with variable growth patterns and cellularity (Figs. 1.33 and 1.34).
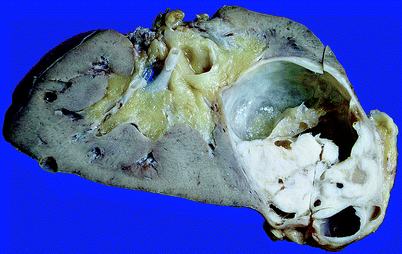
Fig. 1.33
Mixed epithelial and stromal tumor of kidney. The lesion is cystic, but has a prominent solid component (From MacLennan GT, Cheng L. Neoplasms of the kidney. In: Bostwick DG, Cheng L, editors. Urologic Surgical Pathology. 2nd ed. Edinburgh: Mosby/Elsevier; 2008, with permission)
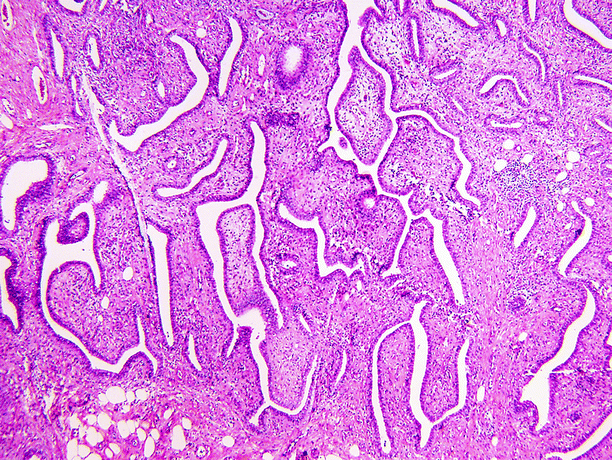
Fig. 1.34
Mixed epithelial and stromal tumor of kidney (MESTK). MESTK is a biphasic tumor, with spindle cell and epithelial components, both of which show a wide spectrum of histological findings. The spindle cell component typically varies considerably in cellularity. Fat cells may be present (lower left). The epithelial component consists of variably sized glands of variable shape and size or tubular structures with complex branching or papillae. Neither the spindle cell nor the epithelial components show significant cytologic atypia or mitotic activity
The great majority occur in females, with a 10:1 predominance and with a mean age of 46 years.
Although the majority present with hematuria, flank pain, or symptoms related to urinary infection, some are discovered incidentally.
It bears many clinical and pathological similarities to cystic nephroma, and it has been suggested by some investigators that these entities represent a spectrum of the same entity, with a variable stroma-cyst ratio. This issue is currently unresolved.
Malignant Renal Neoplasms
Classification of malignant renal neoplasms is summarized in Table 1.3.
Table 1.3
Types of malignant renal neoplasms
Renal cell carcinomas | Nephroblastic neoplasms | Malignant mesenchymal renal neoplasms |
|---|---|---|
Clear cell renal cell carcinoma | Nephroblastoma (Wilms’ tumor) | Clear cell sarcoma of kidney |
Multilocular cystic renal cell carcinoma | Cystic partially differentiated nephroblastoma | Leiomyosarcoma |
Papillary renal cell carcinoma | Rhabdoid tumor | |
Chromophobe renal cell carcinoma | Lymphoma | |
Collecting duct carcinoma | Metastasis | |
Renal medullary carcinoma | ||
Translocation carcinoma | ||
Mucinous tubular and spindle cell carcinoma |
Renal Cell Carcinoma (RCC)
General Information
RCC is the most common primary renal cancer, accounting for about 86 % of all primary malignant renal parenchymal neoplasms.
Usually seen in the adult population, after 40 years.
Predisposing factors of RCC include smoking, obesity, hypertension and antihypertensive drugs, occupational exposure (asbestos, cadmium, benzene, trichloroethylene), drugs (phenacetin, diuretics), chronic renal disease, sex (males > females), and race (African American > Whites).
Four percent of RCCs are familial.
Imaging
Ultrasound
Sonographic appearances of RCC vary according to the stage and histological type of the tumor.
Ultrasound has very low sensitivity in diagnosing tumors less than 3 cm in size.
Most common appearance is of a round, isoechoic parenchymal mass which usually results in a contour abnormality (Fig. 1.35a–c).
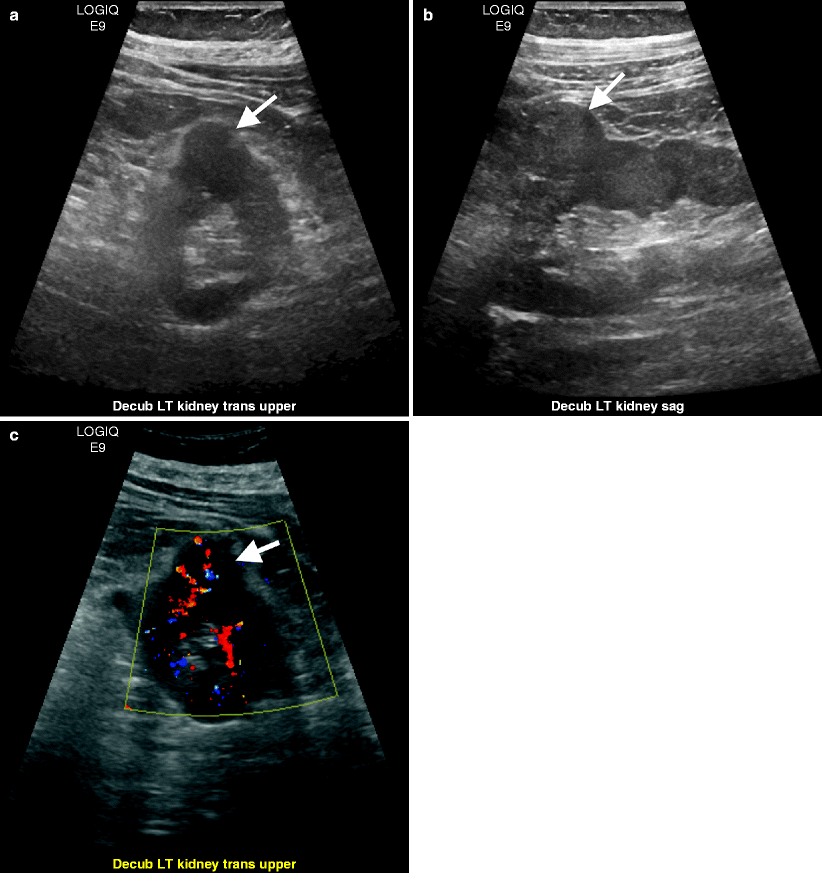
Fig. 1.35
Renal cell carcinoma. Transverse (a) and longitudinal (b) images of gray-scale ultrasound demonstrate an isoechoic left renal mass (arrows). (c) Color flow Doppler ultrasound reveals vascularity in the mass (arrow)
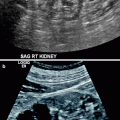
Some tumors may appear heterogeneous with cystic areas secondary to necrosis (Fig. 1.36a).
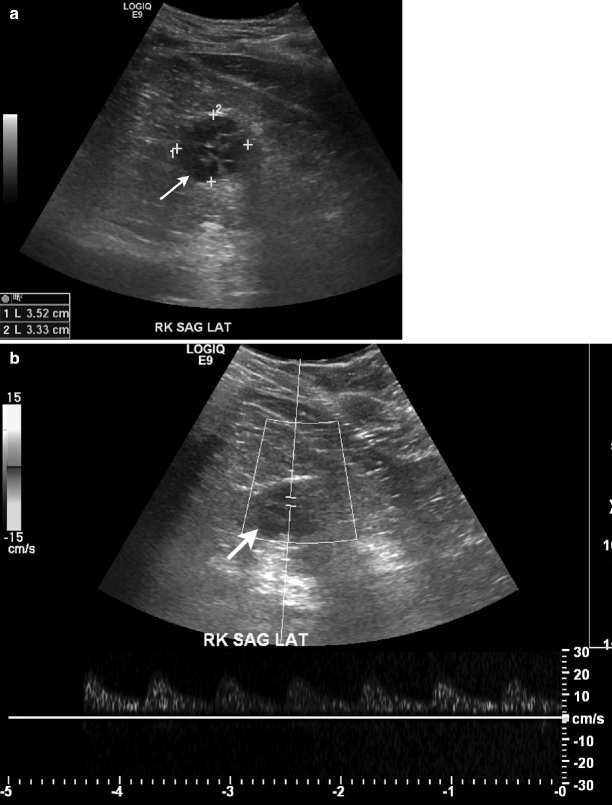
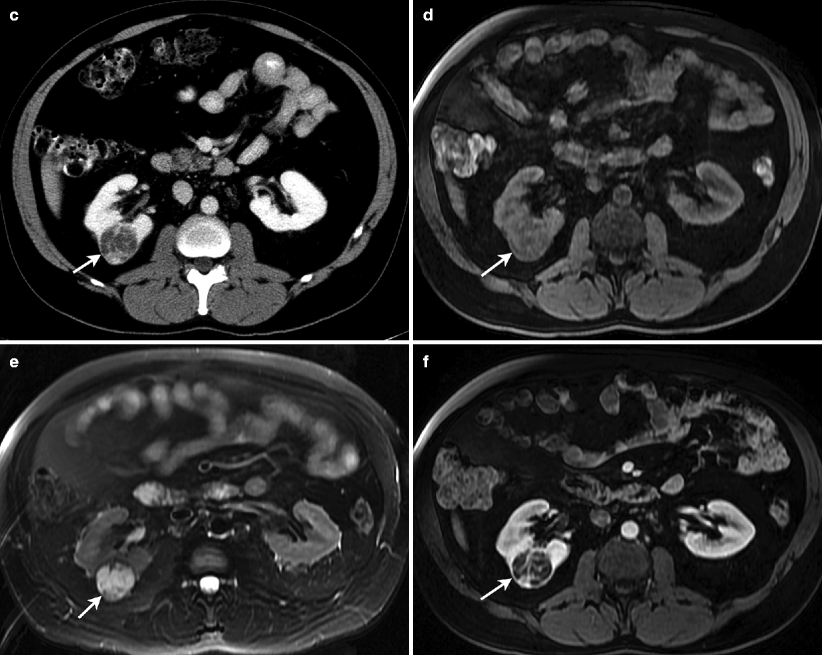
Fig. 1.36
Renal cell carcinoma. (a) Gray-scale ultrasound demonstrates a hypoechoic cystic mass (arrow) in the right kidney with multiple septa. (b) Spectral Doppler reveals arterial vascular flow in the mass. (c) Axial contrast-enhanced CT of same patient demonstrates a well-defined mass (arrow) in the posterolateral aspect of right kidney. Mass lesion enhances mildly and appears hypodense relative to the renal parenchyma. Lesion appears hypointense on T1-weighted (d) and hyperintense on T2-weighted (e) images. (f) Contrast-enhanced T1-weighted image reveals mild enhancement in the mass
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree