© Springer International Publishing Switzerland 2016
Ardeshir R. Rastinehad, David N. Siegel, Peter A. Pinto and Bradford J. Wood (eds.)Interventional Urology10.1007/978-3-319-23464-9_2929. Retroperitoneal Biopsy: Indications and Imaging Approach
(1)
Interventional Radiology Section, Radiology and Imaging Sciences Department, National Institutes of Health Clinical Center, Bethesda, MD, USA
Keywords
Retroperitoneal biopsyImaging the retroperitoneumBiopsy of the retroperitoneumLiposarcomasLeiomyosarcomasPrimary retroperitoneal neoplasmsSarcomaRhabdomyosarcomaMalignant nerve sheath tumorHodgkin’s and non-Hodgkin’s lymphoma of the retroperitoneumThe contents of the retroperitoneum are defined by the boundaries of the potential space behind the posterior abdominal parietal peritoneum and the fascia investing the lumbar musculature. Primary retroperitoneal neoplasms are a rare group of tumors which do not arise from a specific organ but rather originate from tissues or rests of embryonic cells which exist in the retroperitoneum [1]. The most common variety is sarcoma, which accounts for up to 90 % of lesions after lymphoma is excluded. Liposarcomas and leiomyosarcomas are the next most common types, accounting for up to 15 % of tumors. The average age of presentation is during the fifth to seventh decades, and the tumors are often large in size at diagnosis due to the paucity of symptoms associated with growth of retroperitoneal tumors in general. Excluding lymphomas, the most frequent primary retroperitoneal malignancies in decreasing order include liposarcoma, MFH, leiomyosarcoma, rhabdomyosarcoma, and malignant nerve sheath tumors [2]. Both Hodgkin’s and non-Hodgkin’s lymphoma may also occur in the retroperitoneum. Epithelial tumors may rise from the kidney, adrenal gland, and pancreas, and metastatic disease from germ cell tumors, primary carcinomas, or melanomas can also occur. Benign tumors may have neurogenic origins as well (schwannomas, neurofibroma, paraganglioma) [3].
Retroperitoneal tumors are diagnosed at physical examination if they are particularly large, or commonly by imaging when the patient presents with insidious onset of non-localizing symptoms such as lower extremity or genital edema, weight loss, anorexia, urological symptoms, or back pain.
Imaging of Retroperitoneal Tumors
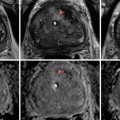
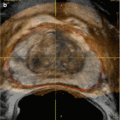
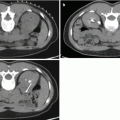
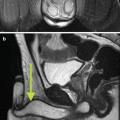
Precise localization and compartmentalization of large masses can be difficult as the size the mass obscures its focus of origin. Displacement of anatomic structures may help to localize the origin. Cross-sectional imaging including MRI and CT provides a complete overview of the peritoneal cavity and retroperitoneal spaces. MRI imaging can exquisitely “characterize” retroperitoneal masses [4], although no specific tumor histology features unique or diagnostic imaging characteristics by virtue of shared tissue components. At the same time, these common tumor tissue components may provide important clues to the origin of large retroperitoneal tumors, including fat signal associated with lipoma, liposarcoma, and teratoma, and myxoid stromal seeding in neurogenic tumors as well as myxoid liposarcomas and malignant fibrous histiocytoma.
A concise review of the imaging of retroperitoneal masses is provided by Rajiah et al. [5]. Additional radiographic signs which may aid in the identification of mass origin include beak sign, embedded organ sign, and embedded organ sign [2]. Nishino et al. [2] also summarize patterns of tumor spread around and between normal structures which may provide clues to retroperitoneal tumor origins, e.g., tumor extension along and around normal structures as a characteristic of tumors of sympathetic ganglia origin [6]. Viable tumor often shows some degree of enhancement after the administration of intravenous contrast material, while necrotic material shows reduced density, hyperintensity on T2W imaging, and absence of contrast enhancement. Fat-containing tumors show high signal intensity on T1-weighted MR imaging, as well as corresponding loss of signal on fat-suppressed image sequences. Myxoid stroma characteristically appears hyperintense on T2W MR imaging and may show delayed enhancement after gadolinium injection [6]. Teratomas may feature fluid attenuation, fat-fluid levels, and calcifications [7]. Neurogenic lesions such as schwannomas typically appear hypointense on T1W and T2W noncontrast imaging and exhibit heterogeneous patterns of contrast enhancement [5]. Malignant fibrous histiocytoma displays heterogeneous signal characteristics on all MR pulse sequences [4].
Benign retroperitoneal masses include lymphangioma, lipoma, myelolipoma, angiomyolipoma, lipoblastoma, hibernoma, nerve sheath tumors, and paraganglioma. Lymphangiomas have a unilocular or multilocular cystic appearance and are diagnosed in infancy, while lipoblastomas typically present in childhood or teenage years. Hibernoma is a rare tumor composed of fetal or brown fat which is most frequently diagnosed in the fourth decade of life. Lipomas rarely occur in the retroperitoneum. These tumors typically grow rather slowly and present as large retroperitoneal masses whose radiographic appearance is characterized by their fat content. These tumors must be distinguished from pelvic lipomatosis as well as liposarcoma; even pathological diagnosis of lipoma should be suspected as under sampled liposarcoma [8].
Myelolipoma is also characterized by an abundance of adipocytes but more commonly arises from the adrenal glands. Extra-adrenal examples are exceedingly uncommon and may be misinterpreted at biopsy [9]. Extrarenal angiomyolipoma is an extremely rare tumor which typically presents as incidental findings during investigations for other purposes, or with abdominal pain and hemorrhagic shock [10].
Paragangliomas, the extra-adrenal equivalent of pheochromocytomas which arise from residual adrenal medullary chromaffin cells, are most commonly found in proximity to the aorta and sympathetic ganglia. Malignant paragangliomas may be difficult to distinguish from benign lesions, with malignancy established by the recognition of local invasion or metastases.
Peripheral nerve sheath tumors comprise another group of benign retroperitoneal neoplasms. The most common peripheral nerve tumor is the schwannoma, which is typically discovered as a large, well-circumscribed mass featuring cystic degeneration.
Lymphoma is the most common form of retroperitoneal malignancy. Non-Hodgkin’s lymphoma tends to involve a larger variety of lymph node groups than Hodgkin’s lymphoma. Malignant lymph nodes may show moderate homogeneous to patchy inhomogeneous enhancement postgadolinium administration. In contrast to metastatic lymphadenopathy, primary lymphoma usually does not demonstrate nodal necrosis. [Retroperitoneal fibrosis after lymphoma therapy may be difficult to distinguish from but fibrosis most likely has low T2W signal]. Retroperitoneal lymphadenopathy is typically seen in other nonneoplastic conditions such as mycobacterium avium-intracellulare infection (MAI) or Castleman’s disease (giant lymph node hyperplasia).
Liposarcoma is the most common malignant primary retroperitoneal neoplasm. Although difficult to distinguish from benign lipoma, the presence of proportionally larger nonadipose components and greater enhancement of septations may be more suggestive of malignancy.
The retroperitoneum is the second most common site for malignant fibrous histiocytoma, the most common type of adult soft tissue sarcoma [11]. This tumor is frequently associated with invasion of adjacent organs, together which large size conveys a poorer prognosis. Calcification has been identified in 7–20 % of lesions [5]. Solid lesions often demonstrate a peripheral nodular enhancement pattern or “pseudocapsule” on precontrast MRI imaging [4].
Germ cell tumors uncommonly originate in the retroperitoneum and are more commonly observed in men [5]. Rajiah et al. [5] further observe that extragonadal germ cell tumors are often observed in the midline between the T6 and S2 vertebrae and that a mass in this location is more suggestive of a primary extragonadal germ cell tumor than metastasis.
Biopsy Indications and Image-Guided Approach
Diagnosis of retroperitoneal tumors often requires tissue sampling; Strauss et al. [3] propose that for patients with (1) retroperitoneal tumors for which diagnosis is uncertain from the radiological appearance, (2) histologies for which neoadjuvant therapy may be appropriate as induction therapy, and preoperative biopsy is “mandatory.” Surgical tissue sampling is invasive and associated with fixed morbidity [12]. In the past, fine needle aspirations were recommended primarily to reduce the risk of hemorrhage or injury to adjacent organs, but the safety and efficacy of cutting needles has been firmly established [13]. Image-guided percutaneous biopsy has been shown to provide satisfactory yield with reduced morbidity and mortality [14]. Tissue sampling is also important to characterize retroperitoneal lesions for therapy planning.
Biopsy needle selection has been based primarily upon efficacy of tissue sampling for lymphoma. Knelson et al. [15] reviewed CT-guided needle biopsy for retroperitoneal lesions, and both the diagnosis and the histological subtyping of lymphoma could be determined in 10 of 11 cases using the 14-gauge Tru-Cut needle, but it was not possible to make the specific diagnosis in any of the lymphoma patients using the 20-gauge Chiba needle. Agid et al. [16] reported that CT-guided core needle biopsies were sufficient to establish a diagnosis in 83 % of the patients with lymphoproliferative disorders and they suggested that it should be used as the first step in the diagnosis of lymphomas. Stattaus et al. [17] reported that the correct lymphoma subtype could be revealed for retroperitoneal masses in 87 % of the patients by using a 16- or 18-gauge core biopsy system with the coaxial technique under CT guidance. In the study of Tomozawa et al. [18], 43 (96 %) of 45 patients had a defined diagnosis with the correct histological subtype determined with an 18-gauge core needle, and subsequent treatment was performed on the basis of the biopsy results. Although needle size will be best determined by suspected histology as well as local expertise, most core needle biopsies performed with 18-guage needles will provide diagnostic material.
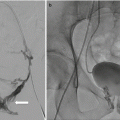
Imaging guidance is often provided by static CT imaging, CT fluoroscopy, cone beam CT, or ultrasound, although the specific guidance modality is often dictated by operator preference. The simplest and preferred biopsy path trajectory is often a straight line to the tumor target from the skin entry site in a single axial plane. Off-plane approaches are possible and often aided by combinations of imaging systems and biopsy planning software packages. UItrasound, CT fluoroscopy, and cone beam CT packages which link CT coordinates to the active fluoroscopic image can provide real-time visualization of the biopsy needle in the trajectory to the target lesion. These advantages may shorten procedure time, reduce nontarget punctures, and reduce radiation exposure [19].
Ultrasound guidance enjoys the advantage of avoiding patient exposure to ionizing radiation. Ultrasound images of the retroperitoneum are generated based upon the differential ability of tissue to reflect or transmit sound of frequency between 3.5 and 7 MHz in the clinical realm. Ultrasound permits the operator to monitor needle placement in real-time fashion without ionizing radiation as metallic needles are sonoreflective. In addition, sonography with color Doppler technology can identify significant intra-tumoral vascularity to be avoided by needle puncture. Deep abdominal and pelvic targets are best imaged with a curved array 3.5–5 MHz probe, while more superficial targets can be imaged with improved resolution with 5 MHz and greater linear array probes. Needle guidance can be accomplished “freehand” or with the use of specifically designed needle guides which attach to the transducer. Ultrasound delineation of retroperitoneal masses may be obscured by overlying bowel gas, necessitating placing the patient in a lateral decubitus or prone position for visualization. Yarram et al. observed an ultrasound-guided pelvic mass biopsy success rate of 95.4 % compared with 84.6 % for CT guidance [20].
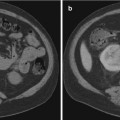
A pre-procedure CT or MRI scan can provide a basis for biopsy path and target planning. In general, the planned needle biopsy path should exclude the viscera, the pleura, and the visible blood vessels. Other structures to be avoided which are inconstantly imaged or not visualized include the ureters and sciatic and genitofemoral nerves. In some instances, target selection must be refined toward viable regions of tumors as opposed to more necrotic regions. Tumor viability is indirectly evidenced by increased soft tissue density comparable to the muscle as well as observed enhancement following the administration of vascular contrast material (CT or MRI). CT showing low-density tissue in the central portion of the tumor may be related to liquifactive or hemorrhagic necrosis and should be avoided during tissue sampling. Leiomyosarcomas in particular may demonstrate significant zones of necrosis [21]. Hypervascularity may be recognized according to the presence of adjacent discrete blood vessels, increased density following vascular contrast administration (CT), or by noting increased blood flow within portions of the lesion according to color Doppler sonography. Characteristic tissue components not only aid in radiographic diagnosis but also may inform biopsy planning; liposarcomas will have varying amounts of fat, with high-grade liposarcomas having the least amount. Tissue sampling of suspected liposarcoma from areas of increased tissue density may increase diagnostic yield. Myxoid tissue has well-recognized specific MR imaging characteristics [6]. Neurogenic tumors more typically contain myxoid tissue, although liposarcomas and myxoid malignant fibrous histiocytoma may also exhibit myxoid tissue [5].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree