Fig. 1
ExAblate MRI table. The table will dock with the MRI scanner. The ultrasound transducer can be seen in the middle of the table. (Image courtesy of InSightec)
Fig. 2
The inner workings of the ExAblate equipment that shows the ultrasound transducer connected to cables that help control the positioning of the transducer, (Image courtesy of InSightec)
The relatively small size of the individual focal points and the cooling intervals required between sonications means that long treatment times are needed to treat fibroids. The Sonalleve system has a phased array 256 channel transducer and the focus of the HIFU beam is electronically steered along a trajectory that comprises multiple outward-moving concentric circles (Kim et al. 2012a, b). This creates a volumetric ablation in which the system can provide four differently sized ellipsoidal treatment cells that are 4, 8, 12, or 16 mm in the axial dimension and 10, 20, 30, or 40 mm in the longitudinal dimension, respectively (Kim et al. 2012a, b). This allows treatment volumes of 0.08, 0.67, 2.26, and 5.36 mL, respectively (Kim et al. 2012a, b). The larger volume of ablated tissue should potentially reduce treatment time. The ExAblate 2100 system allows the transducer to come closer to the abdominal wall, decreasing the energy density on the patient’s skin (Trumm et al. 2013). Larger sonication spots (up to 70 mm; previously 45 mm) allow ablation of larger fibroid volumes (Trumm et al. 2013). The maximum energy level of the sonications has also been increased to 7,200 J (Trumm et al. 2013). There is also 3D treatment planning software that helps to reduce total treatment time (Trumm et al. 2013). All of these changes are attempts to increase the speed and effectiveness of the ablation.
The application of high intensity focused ultrasound causes an increase in the temperature of the tissue in the focal area. When the temperature elevation is large enough and maintained for an adequate period, tissue damage will result (Hynynen 2008). To create thermal damage, the exposure of the tissue to a given temperature has to exceed a threshold time. If the temperature/time does not exceed the threshold, the tissue may recover. When the temperature in the target is raised to an appropriate level, protein denaturation occurs, resulting in cell death and creation of a coagulation necrosis. The tissue in the path of the ultrasound beam, away from the focus, is warmed, but only to sub-lethal temperatures (Catane et al. 2007). Although a certain measured energy may be put into a tissue, the temperature elevation is not necessarily the same in all tissues. The type of tissue, the presence of large blood vessels that act as a heat sink, the size, and shape of the ultrasound field all influence the temperature elevation achieved (Hynynen 2008).
2.2 MRI Temperature Measurement Principles
MRI has important properties for monitoring the focused ultrasound procedure. MRI has excellent soft-tissue contrast and the ability to provide fast, quantitative temperature imaging in a variety of tissues (Stafford and Hazle 2008). The proton resonance frequency shift (PRF) of water changes in response to changes in temperature (Tempany 2007). For both the ExAblate and Sonalleve equipment, real-time thermal mapping at the target site is achieved using phase imaging on the basis of the shift in PRF caused by temperature rise (Catane et al. 2007; Voogt et al. 2012a, b). Phase-difference fast-spoiled gradient-echo MR imaging, or “phase map” imaging, is performed at the targeted region before, during, and immediately after sonication (Tempany 2007). These images are used to construct the temperature images acquired during the sonications (McDannold et al. 2006). Those images are automatically compared with a reference image obtained immediately before the sonications to create a real-time thermal map (Catane et al. 2007). The benefit of combining MR with the focused ultrasound treatment is real-time monitoring of the localization of the individual sonications, enabling the measurement of energy deposition and the temperature changes in the region being treated, and feedback on the effectiveness of the sonications (Tempany 2007). The Sonalleve system provides the option of using the online acquired temperature information for automatically controlling the sonication using a thermal feedback method which stops the sonication when the measured thermal ablation and temperature profile match with that intended for the chosen treatment cell (Voogt et al. 2012a, b; Venkatesan et al. 2012).
3 Candidates for MR Guided Focused Ultrasound
The patient should have symptoms referable to fibroids and should be prescreened with an MRI. The prescreening MRI should be performed in the prone position, which gives a better idea of the positioning of the uterus/fibroids when the patient is in the treatment position. There should be a limited number of fibroids preferably 1–4 since each of the fibroids will require treatment and large numbers of fibroids will make the time of treatment prohibitive. The size of a single fibroid should usually be under 10 cm and usually more than 900 ml of total fibroid volume is an exclusion. Fibroids that are homogeneous and hypointense (dark) on T2 seem to respond better than fibroids that are heterogeneous and hyperintense (bright) on T2 (Funaki et al. 2007; Tempany 2007). Fibroids should be enhancing since if they have degenerated/infarcted (lost their blood supply) there is no reason to treat them.
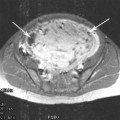
Patients cannot have contraindications to MR imaging such as cardiac pacemakers, sensitivity to MR contrast agents, severe claustrophobia, or patients who exceed the size limitation of the MRI scanner. Abdominal scarring (Fig. 3) or bowel loops in the path of the ultrasound beam are contraindications, unless they can be displaced from the beam path; (Yoon et al. 2011) patients with intrauterine device in place should have the IUD removed. The presence of IUDs has been an exclusion criteria. Potentially if the IUD is completely out of the path of the ultrasound beam, it might be possible to treat the patient. Patients with diffuse adenomyosis and no fibroids probably should not be treated, although treatment of focal adenomyosis/adenomyomas has been described (Rabinovici et al. 2006; Fan et al. 2012; Polina et al. 2012). Patients with both adenomyosis and fibroids should be counseled that although their fibroids may be able to be treated, the adenomyosis may be the cause of symptoms. Obese patients may have so much subcutaneous tissue that the fibroid is largely out of the range of the ultrasound beam. If the fibroid or the majority of the fibroid is beyond the ultrasound’s focal distance then an adequate treatment will not be possible.
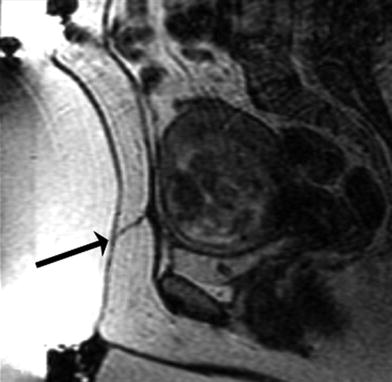

Fig. 3
Screening MRI demonstrates a scar (arrow) from a previous Caesarian section. This scar is directly in the path of the ultrasound beam. Treating in the vicinity of a scar may lead to severe burns and is a contraindication for MRgFUS treatment
Patients cannot be pregnant, and should not have any other pelvic pathology (active pelvic inflammatory disease, pelvic carcinoma, undiagnosed vaginal bleeding, etc.) that requires treatment or further investigation. Patients with a history of a prior UAE should be assessed with caution because of concern that interaction between the ultrasound beam and the particles used for UAE may lead to a poor result or potentially cause complications if the beam is scattered by the particles.
Determining the patients who might be candidates for the procedure is somewhat difficult. A number of patients (37 %) who were symptomatic with uterine were excluded because they did not meet the clinical criteria for entrance into a study (Arleo et al. 2007). However, if there are no study criteria to be fulfilled then potentially 100 % of patients could be candidates (Zaher et al. 2009). Not all women, even if they are clinically candidates for the MRgFUS procedure are anatomically suitable for the procedure. Approximately 25 % (Zaher et al. 2009) to 75 % (Stewart et al. 2006; Arleo et al. 2007) of patients clinically eligible are not anatomically eligible. The reasons for exclusion include a high fibroid volume, bowel in the path of the beam, significant adenomyosis, pedunculated fibroids, small or nonexistent fibroids, degenerative or infarcted fibroids, bright T2 weighted fibroids, or arteriovenous malformations, or calcified fibroids (Arleo et al. 2007; Zaher et al. 2009).
4 MR Guided Focused Ultrasound Procedure
The MRgFUS procedure is an outpatient procedure. Patients come in early in the morning, and have the procedure and are discharged once they have recovered from their sedation, usually about 30 min after having finished the procedure.
The patient is asked to fast over night, and in some cases when the position of the bowel is considered problematic, the patient maybe asked to follow a low residue diet for a few days prior to the procedure. The skin on the lower abdominal wall from the umbilicus to the pubic symphysis is shaved to prevent any air bubbles being trapped in the hair, which would interfere with the ultrasound beam and potentially increase the risk of skin burns. The skin is cleaned with alcohol to remove any lotion, oils, or powder on the skin that might put the patient at risk for burns. An intravenous catheter is placed so that the patient can be given moderate sedation and any other medications that might be required. A Foley catheter is placed since the patient will be undergoing the procedure for up to 4 h and it is important to be able to keep the bladder empty since filling of the bladder will displace the uterus and change the positioning of the fibroids.
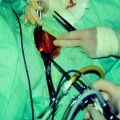
The procedure is performed with the patient lying in a prone position on the treatment table with her pelvis positioned over the transducer. Her abdomen is in a water bath, with degassed deionized water, in contact with an acoustic gel pad (Fig. 4). The patient is positioned with her feet toward the MRI chamber allowing her to look out into the room, which has the benefit of reducing claustrophobia. The patient is usually given conscious sedation to help her relax during the procedure.
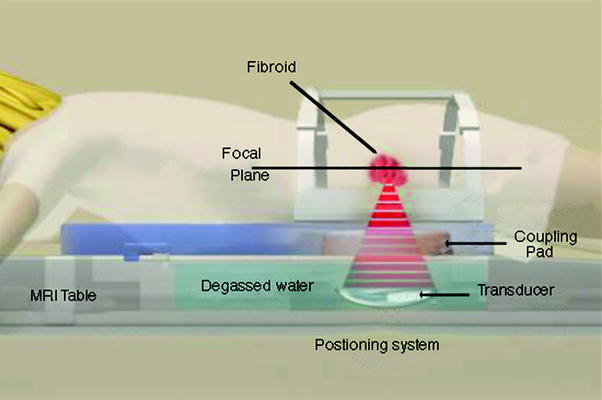

Fig. 4
Patient in position on the MRgFUS table. She is lying in the water bath on top of an acoustic gel pad that allows good contact between the transducer and the body
The treatment planning begins by obtaining localizer images to determine if the patient is properly positioned with the uterus over the transducer (Fig. 5). The treatment area is defined by the radiologist, and the target volume is analyzed with superimposition of ultrasound beam paths in all three planes (Fig. 6). The energy beam pathway is evaluated to avoid any structures that would be in the path such as bowel, pubic bone, bladder, or nerves. No sonication should be performed within 4 cm of a bony structure in the far field of the beam. The path is also examined to make sure that there are no scars, surgical clips, or air bubbles that might cause ultrasound aberrations. The number and position of sonications are planned to encompass the entire target volume. With the Sonalleve equipment the location, size, and number of treatment cells are chosen and positioned on the 3D planning images (Kim et al. 2012a, b). The ultrasound transducer beam can be tilted in multiple directions in order to avoid bowel, pubic bone, or sacrum.
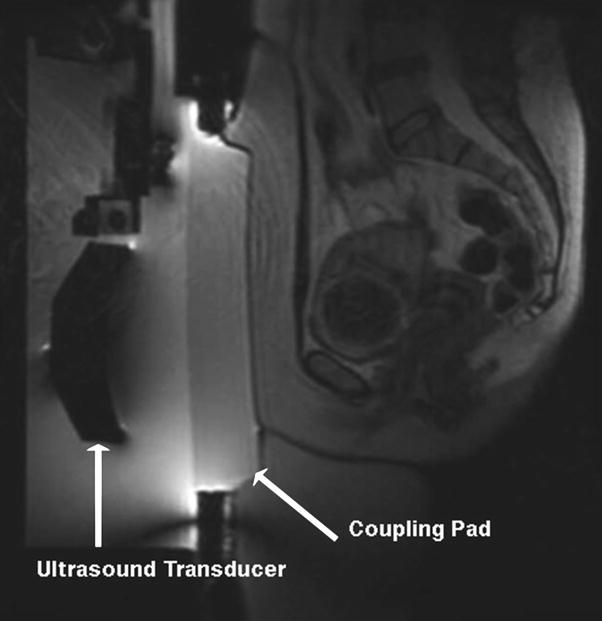
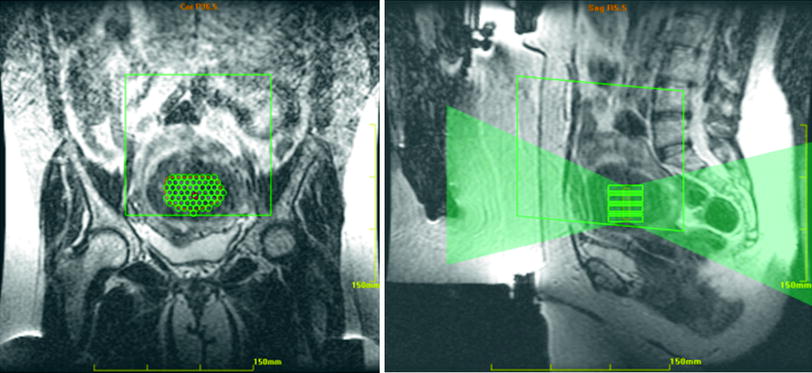

Fig. 5
A preliminary scan that demonstrates the ultrasound transducer, and the coupling pad. Ideally, the fibroid should be directly opposite the concave portion of the transducer. This positioning is very good

Fig. 6
The treatment area has been drawn in the center of the fibroid. The small green circles within the treatment area define the number and position of proposed sonications. On the sagittal image, the fan of green shows the planned sonication beam path. Note that the patient is positioned somewhat below the transducer, ideally she should be moved up slightly to get the fibroid opposite the transducer
If the fibroid is at the serosal surface of the uterus, a 0.5 cm margin of non-targeted tissue should be maintained. This is to prevent the possibility of thermal damage of tissues in close proximity to the serosal surface of the uterus. If there is bowel anterior to the uterus, positioned between the abdominal wall and the uterus, the bladder can be filled using the Foley catheter, and this will elevate the uterus and may displace the bowel. Depending on the depth of the fibroid, it may be possible to treat through the distended bladder. Otherwise the bladder is emptied, and in some cases the bowel may remain displaced above the uterus allowing the treatment to proceed. If the bowel again moves into a position anterior to the uterus, a rectal tube (barium enema equipment) can be placed and after the bladder is again distended, the colon can be filled with water, which causes the uterus to be shifted anteriorly. Then when the bladder is emptied, the pressure on the uterus by the recto-sigmoid may allow the uterus to push the bowel out of the way.
The patient is given a “panic button” to hold during the treatment that allows her to stop the equipment if she experiences severe pain or heating during the procedure. She is asked to be particularly aware of burning sensation on the abdominal wall, or of pain radiating down her legs. The patient is also made aware that the procedure is not painless. Most patients experience a cramping sensation during each sonication. Some patients have described the sensation as “back labor”. Some sonications have more discomfort than others, depending on the area of the fibroid being treated, and depending on the energy, power, spot size, and other parameters (Fig. 7).
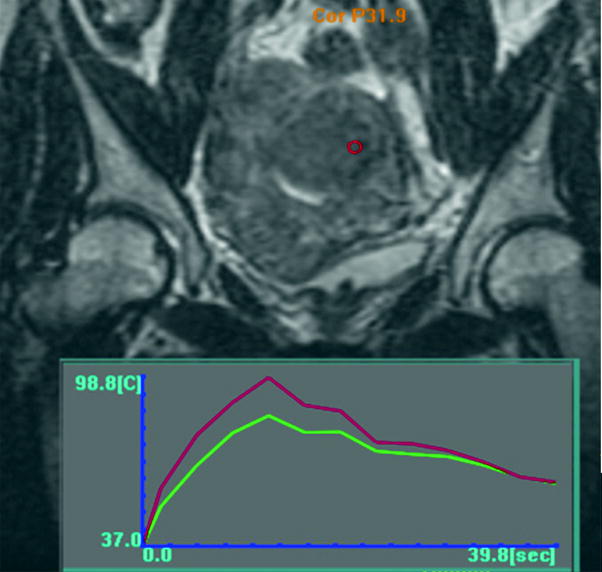

Fig. 7
The temperature graph of a sonication. The sonication spot is shown as a red circle on the fibroid. The superimposed graph show that there was a 40 s sonication that reached a maximum temperature of 98.8 °C. This temperature is somewhat high; ideally the temperature would peak at 70–80 °C
Procedure time is variable depending on the size, and number of the fibroids being treated, and also depending on the amount of time needed to position the patient, and potentially to mitigate the position of bowel or scar. Most patients do not tolerate more than about 4 h in the scanner which was the maximum allowed in the clinical trials in the US. Variable times have been reported with procedure times ranging from about 2–7 h with a mean of about 4 h in some recent studies (Trumm et al. 2013; Kim et al. 2012a, b).
Following the treatment, fat-saturated, T1-weighted contrast enhanced images are obtained in the sagittal, coronal, and axial planes to evaluate the extent of the ablated area. The volume of ablated tissue can then be calculated, and a percentage of treated versus non-treated fibroid tissue can be determined (Fig. 8). If there remains a substantial amount of perfused (non-treated) tissue, the patient may undergo a second treatment to try and ablate the remaining viable fibroid tissue.
Get Clinical Tree app for offline access

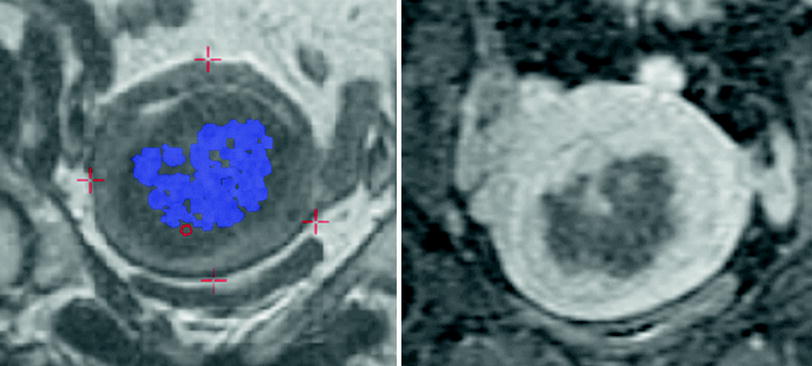
Fig. 8
The image on the left demonstrates the accumulated thermal dose seen represented by the blue dots. Ideally these blue dots




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree