Fig. 8.1
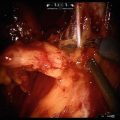
Incising the peritoneum lateral to the right medial umbilical ligament down to the level of the vas deferens and internal ring on the right
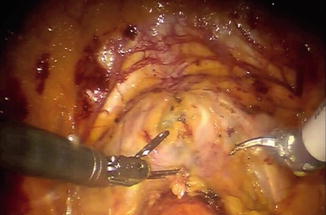
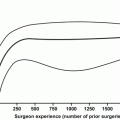
Fig. 8.2
Markedly fibrotic and thickened endopelvic fascia in a patient undergoing sRALP after external beam radiation therapy
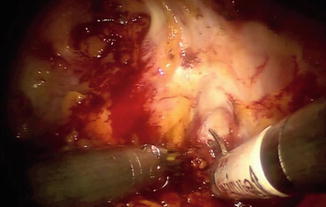
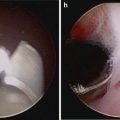
Fig. 8.3
Intraoperative view demonstrating incision of the thickened right endopelvic fascia
The bladder neck is then identified and incised with a combination of sharp dissection and electrocautery as per a standard RALP (Fig. 8.4). Once the posterior bladder neck has been incised, the vasa deferentia are identified and divided (Fig. 8.5). The fourth arm is utilized to provide anterior tension on the distal vas deferens, elevating the prostate. The third arm can be utilized to grasp the proximal vas deferens to facilitate dissection of the seminal vesicles (Fig. 8.6). 30–35 % of patients undergoing salvage radical prostatectomy will have seminal vesicle involvement so careful and wide excision of the seminal vesicles is prudent [19, 21].
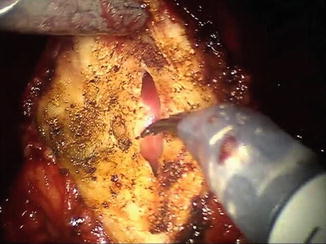
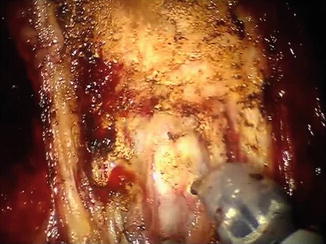
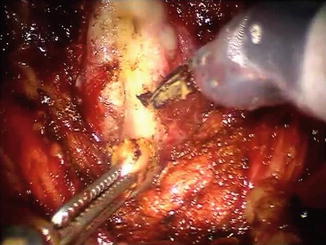

Fig. 8.4
The anterior bladder neck has been incised utilizing a combination of cautery and sharp dissection

Fig. 8.5
The posterior bladder neck is incised and both vasa deferentia are identified

Fig. 8.6
After dissection and division of the right vas deferens, the fourth arm is used to provide anterior retraction on the distal stump (out of the picture) and the third arm utilizes the proximal stump of the vas deferens as a handle to aid in the dissection of the right seminal vesicle
Development of the Posterior Plane and Control of the Pedicle
The plane posterior to Denonvilliers’ fascia is generally well preserved in patients after primary radiation therapy, especially when compared to the interfascial plane above Denonvilliers’ fascia (Fig. 8.7). Dissection in this plane also permits wide excision of the posterior prostate in the case of disease extension. Even locally extensive tumors rarely penetrate Denonvilliers’ fascia. The fourth arm is utilized to retract the prostate and seminal vesicles anteriorly and cranially. Tension is placed on Denonvilliers’ fascia with the third arm and the fascia should be incised sharply, exposing the perirectal fat (Fig. 8.8). This plane should be developed caudally towards the posterior prostatic apex without the use of cautery and is greatly facilitated by the improved visualization afforded by robotic-assistance, which allows an easier and safer posterior dissection (Fig. 8.9). This step should proceed cautiously as dense adhesions may be present. While some have advocated for nerve-sparing sRALP, we generally perform a wide excision of the lateral prostatic fascia and neurovascular bundle in this setting to optimize the chance of cure [15, 17, 19]. 38–45 % of patients undergoing salvage radical prostatectomy will have extra-prostatic extension on final pathologic analysis. Furthermore, erectile function in a post-radiation cohort is often poor even before surgery [19, 21]. The pedicles are then isolated via anterior retraction on the prostate and seminal vesicles and divided with Hem-o-lok clips (Teleflex Medical, Research Triangle Park, NC) and scissors (Fig. 8.10).
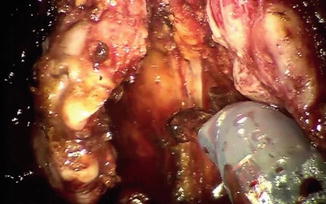
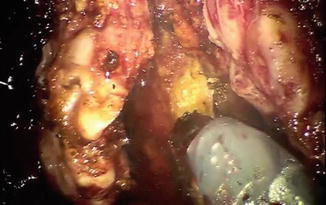
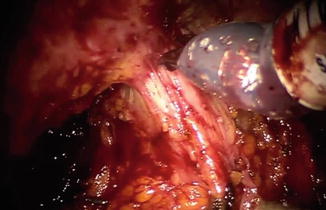
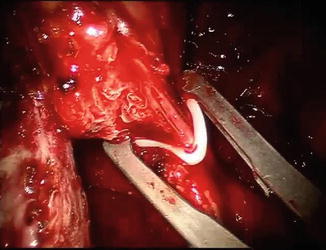

Fig. 8.7
Denonvilliers’ fascia is placed on tension by the third arm and incised sharply. The fourth arm is providing anterior and cranial retraction on the prostate and seminal vesicles (out of the picture)

Fig. 8.8
The perirectal fat is exposed after incision of Denonvilliers’ fascia, indicating the correct posterior plane in the salvage setting

Fig. 8.9
The posterior plane is almost completely developed. The rectum is tented up as the last remaining attachments between the posterior prostatic apex and the rectum are divided sharply

Fig. 8.10
The right prostatic pedicle is isolated and controlled with Hem-o-lok clips (Teleflex Medical, Research Triangle Park, NC)
Apical Dissection and Division of the Urethra
Prior to division of the dorsal venous complex, we will completely free the lateral margins of the prostate to allow full mobilization of the prostate. This improves visualization of the posterior prostatic apex, which is often the most adherent after radiation therapy—particularly with brachytherapy (Fig. 8.11). The fourth arm is used to pull the prostate posteriorly and cranially to aid in the definition of tissue planes which can be quite obliterated and fibrotic. The three-dimensional magnification of the operative field afforded by robotic-assistance is again extremely helpful around the apex. In the salvage setting we do not ligate the dorsal venous complex prior to its division in order to improve mobility and visualization during dissection of the prostatic apex. Bleeding is usually limited due to tissue fibrosis and loss of vascularity as well as the effect of the pneumoperitoneum. Following division of the dorsal venous complex, the urethra is sharply divided, the catheter is removed, and any remaining apical prostatic tissue should be meticulously dissected free (Fig. 8.12). This can be quite difficult in the salvage setting and care must be taken to decrease the chance of a positive apical margin. At times, the apical and periprostatic fibrosis may obscure the true boundaries of the prostate and additional margins can be sent off as needed. After completion of the apical dissection, division of the urethra and posterior attachments, a figure-of-eight with 2-0 polyglactin (vicryl) suture on an SH needle can be placed on the distal dorsal venous complex for hemostatic purposes.
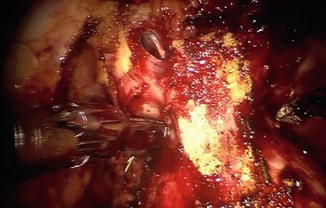
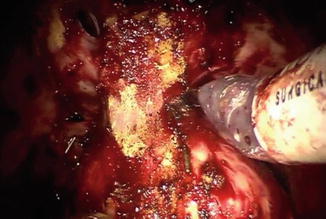

Fig. 8.11
Intraoperative view demonstrating marked apical fibrosis in patient who had previously received brachytherapy

Fig. 8.12
Intraoperative view after division of the dorsal venous complex and urethra demonstrating dissection of the posterior apical prostatic tissue. Note the marked peri-apical fibrosis
Node Dissection
In the significant majority of patients undergoing sRALP, we perform an extended bilateral pelvic lymphadenectomy [19]. The possibility for incremental survival gain and improved staging information must be balanced against the potential of increased perioperative morbidity. Especially when external beam irradiation has been used, there can be considerable fibrosis along the iliac vessels. Although pelvic node dissection is feasible in virtually any patient, the incremental gain in terms of either survival or staging is minimal. Therefore, if node dissection seems excessively hazardous because of scarring from irradiation or prior mesh hernia repair, we may omit it.
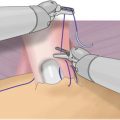
Vesicourethral Anastomosis
Completion of a secure mucosal-to-mucosal anastomosis is critical, especially in the salvage setting. A distinct advantage of the robot is the facility with which a running, water-tight anastomosis can be obtained. This limits postoperative morbidity from a prolonged urine leak, but also minimizes the risk of bladder neck contracture.
The anastomosis is performed with a running single-suture technique using the robotic fourth arm to help monitor tissue approximation. Alternatively, a barbed suture can be used. Regardless, the key is to have complete mucosal-to-mucosal tissue approximation.
Undocking of the Robot
Following completion of the vesicourethral anastomosis, the bladder is filled to assess the integrity of the anastomosis and a surgical drain can be placed through the left lateral robotic trocar incision site. We generally only leave a drain if a lymph node dissection has been performed. The robot is undocked, the table is leveled, and the trocars and specimen are removed. The fascia of the 12 mm assistant port and supra-umbilical camera port sites are closed with #1 absorbable polyglactin (vicryl) suture after utilizing the camera to inspect the anterior abdominal wall and port sites for bleeding. The skin is then closed and the patient is awakened and taken to the recovery room.
Postoperative Care
Patients undergoing sRALP are managed postoperatively according to our standardized robotic prostatectomy pathway, which includes early ambulation, a full liquid diet on the morning of postoperative day 1, intravenous ketorolac to reduce the utilization of narcotics for pain control, and an aggressive bowel regimen [22]. Surgical drains are generally removed on the first postoperative day, unless the output is greater than 200 cc over 24 h, in which case patients will go home with the drain and have it removed once the output approximates this threshold. Salvage status alone should not significantly prolong hospital stay. In our experience, 94 % of patients undergoing sRALP are discharged on the first postoperative day [19]. The Foley catheter is left indwelling for at least 2 weeks because of the poor healing of irradiated tissue. A cystogram prior to catheter removal is performed only if the anastomosis was technically difficult or if there is clinical suspicion of incomplete healing.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree