Scintigraphic Diagnosis of Inflammation and Infection
Christopher J. Palestro
The scintigraphic evaluation of infection and inflammation is extremely broad in scope, encompassing numerous radiopharmaceuticals, imaging techniques, and diseases. In addition to reviewing the roles of gallium-67 (Ga-67) citrate and in vitro radiolabeled leukocytes (white blood cell [WBC]), this chapter also addresses the potential of 18F-FDG-PET (FDG-PET), for imaging inflammation and infection.
Gallium-67
Ga-67, which has been used for localizing infection for more than three decades, is cyclotron produced. It decays by electron capture and has a physical half-life of about 78 hours. With principal photon energies of 93, 184, and 296 keV used for imaging and a poor photon yield per disintegration, Ga-67 is a suboptimal imaging agent (1).
Several factors govern uptake of this tracer in inflammation and infection. About 90% of circulating Ga-67 is in the plasma, nearly all transferrin bound. Increased blood flow and increased vascular membrane permeability result in increased delivery and accumulation of transferrin-bound Ga-67 at inflammatory foci. Ga-67 also binds to lactoferrin, which is present in high concentrations in inflammatory foci. Direct bacterial uptake probably also accounts for some Ga-67 accumulation in infection. Siderophores, low-molecular-weight chelates produced by bacteria, have a high affinity for Ga-67. The siderophore–Ga-67 complex presumably is transported into the bacterium, where it eventually is phagocytosed by macrophages. Although some Ga-67 may be transported bound to leukocytes, it is important to note that, even in the absence of circulating leukocytes, Ga-67 accumulates in inflammation and in infection (1).
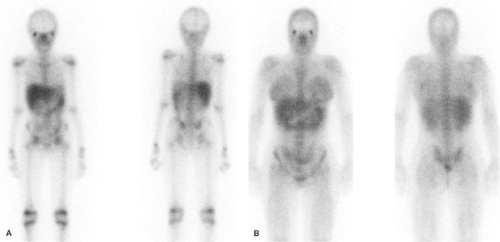
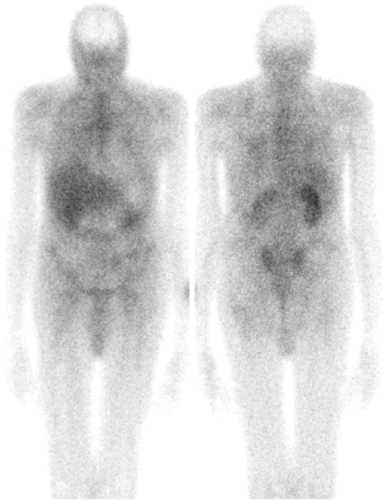
Imaging usually is performed 18 to 72 hours after injection of 185 to 370 MBq (5 to 10 mCi) 67Ga-citrate. A gamma camera, equipped with a medium-energy collimator and capable of imaging multiple energy peaks, is used. The normal biodistribution of Ga-67 is variable and includes bone, bone marrow, liver, GI tract, urinary tract, and soft tissues (2) (Fig. 60.1). Nasopharyngeal and lacrimal gland activity can be very prominent, even in the absence of disease. Intense breast uptake is associated with hyperprolactinemic states including pregnancy, lactation, certain drugs, and hypothalamic lesions. In patients who have undergone multiple transfusions, increased renal, bladder, and bone/marrow activity, together with decreased hepatic and colonic activity frequently, is observed, presumably due to iron receptor saturation by exogenous iron from the transfused cells. The MRI contrast agent gadolinium reportedly causes similar alterations in the biodistribution of Ga-67 (3,4,5,6,7,8).
Although labeled leukocyte imaging is the radionuclide test of choice for imaging inflammation and infection in the immunocompetent population, Ga-67 imaging remains both popular and useful, providing information that is complimentary to, and at times not available from, other tests. Indications for Ga-67 imaging include the following.
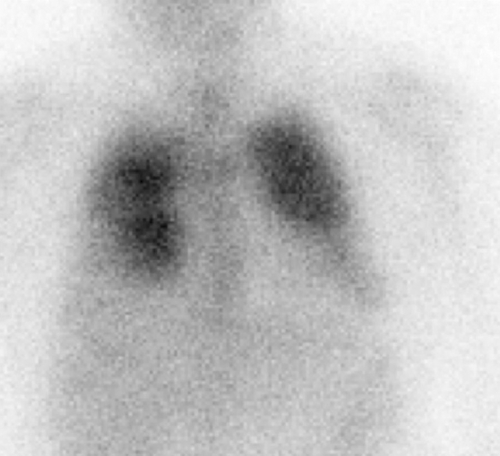
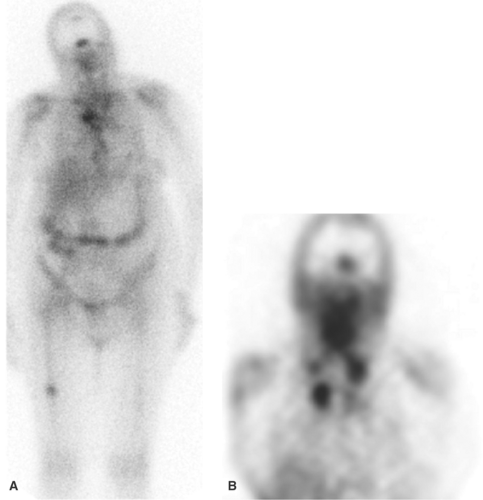
Opportunistic Infection. Nuclear medicine plays an important role in the detection of infections unique to the immunocompromised patient, and for most of these, Ga-67 imaging is the radionuclide procedure of choice. Many opportunistic infections affect the lungs, and a normal Ga-67 scan of the chest excludes infection with a high degree of certainty, especially in the setting of a negative chest radiograph. In the HIV-positive patient, lymph node uptake of Ga-67 most often is associated with mycobacterial disease or lymphoma. Focal, or localized, pulmonary parenchymal Ga-67 uptake usually is associated with bacterial pneumonia. Diffuse pulmonary gallium uptake, especially when intense, is indicative of Pneumocystis carinii pneumonia (Fig. 60.2). In addition to its value for diagnosis, Ga-67 can be used for monitoring response to therapy. Kaposi sarcoma, a malignancy often found in the patient with AIDS is not Ga-67 avid (1,9,10).
Interstitial Lung Disease. Ga-67 is an extremely sensitive indicator of pulmonary inflammation. Uptake of this tracer occurs in sarcoidosis, interstitial pneumonitis in virtually all its forms and etiologies, drug reactions, collagen vascular disease, and pneumoconioses. Although the degree of activity generally correlates with the severity of the underlying illness, a normal study does not exclude very mild inflammation. Furthermore, neither the intensity nor the pattern of uptake is diagnostic of specific illnesses (11,12).
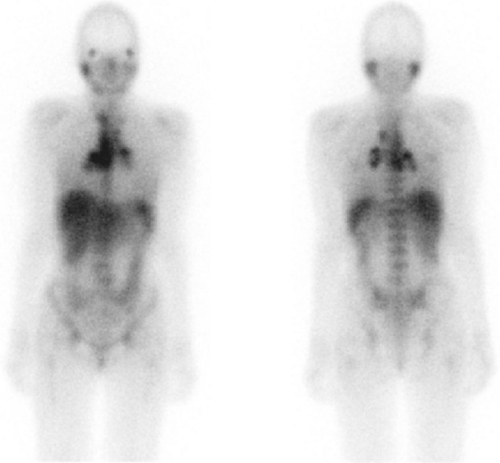
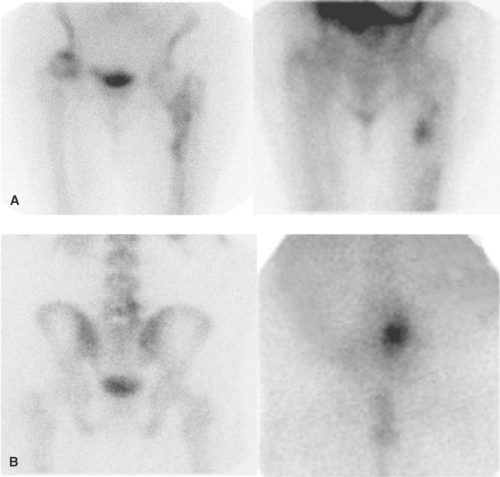
In sarcoidosis, pulmonary uptake of Ga-67 correlates with disease activity and response to therapy. Ga-67 scintigraphy has been reported to be up to 97% sensitive for detection of active sarcoidosis when considering both pulmonary and extrapulmonary sites (Fig. 60.3). Whether Ga-67 imaging
can provide prognostic information or therapeutic insight for other inflammatory lung diseases is not known.
can provide prognostic information or therapeutic insight for other inflammatory lung diseases is not known.
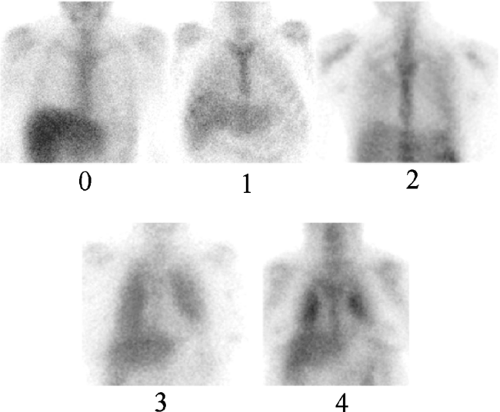
As determining relative pulmonary Ga-67 activity may be helpful for assessing the degree of inflammation, an objective index of Ga-67 activity has been sought. Some authors have chosen to compare pulmonary activity with sternal activity, whereas others have compared pulmonary activity with hepatic activity, and still others have used semiquantitative techniques involving computer acquisitions, SPECT, and whole-body imaging to report activity ratios. There is, however, no standard method for quantifying Ga-67 pulmonary activity. Therefore, when reporting Ga-67 activity, the specific scale and reference standard should be stated. We use a scale of 0 to 4 in which pulmonary activity is compared to liver activity. In this schema, 0 (normal) represents pulmonary activity indistinguishable from background, and 1 represents
equivocally increased activity. Grades 2, 3, and 4 represent definitely abnormal pulmonary activity. Grade 2 is less than, grade 3 is equal to, and grade 4 is greater than hepatic activity. A photopenic or “cold” cardiac silhouette is present in patients with grades 2, 3, and 4 uptake (Fig. 60.4) (1).
equivocally increased activity. Grades 2, 3, and 4 represent definitely abnormal pulmonary activity. Grade 2 is less than, grade 3 is equal to, and grade 4 is greater than hepatic activity. A photopenic or “cold” cardiac silhouette is present in patients with grades 2, 3, and 4 uptake (Fig. 60.4) (1).
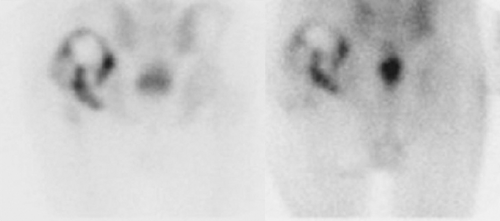
Interstitial Nephritis. Interstitial nephritis, a well-recognized cause of acute renal failure, is characterized by interstitial edema and a mononuclear cellular infiltrate. Although biopsy is required for definitive diagnosis, Ga-67 can be helpful in differentiating interstitial nephritis from acute tubular necrosis in the patient with acute renal failure. Interstitial nephritis is characterized by renal uptake of gallium that is more intense than lumbar spine uptake, whereas acute tubular necrosis is characterized by little or no renal uptake. The test is less reliable in patients with chronic renal failure (Fig. 60.5) (13).
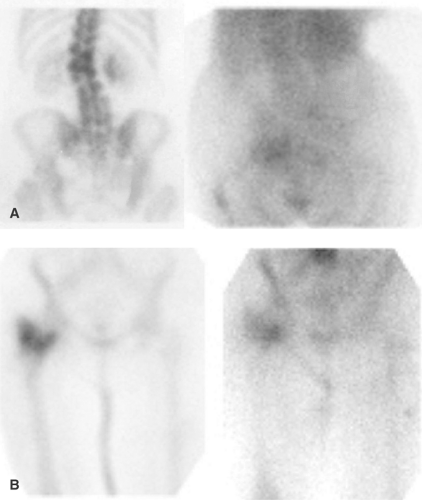
Fever of Undetermined Origin. Fever of undetermined origin (FUO) is an illness of at least 3 weeks duration, with several episodes of fever exceeding 38.3°C and no diagnosis after an appropriate inpatient or outpatient evaluation. The etiologies of FUO are numerous; the most common causes are infections, malignancy, and collagen vascular disease. Other etiologies include granulomatous diseases, pulmonary emboli, cerebrovascular accidents, drug fever, and, occasionally, factitious fever. Radionuclide imaging typically is reserved for those situations in which other imaging tests fail to localize the source of the fever. Nearly 80% of FUOs are due to an entity other than infection, and therefore, Ga-67, which accumulates in infection, inflammation, and tumor, often is preferred over WBC imaging for this indication (Fig. 60.6) (1,2).
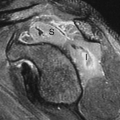
Spinal Osteomyelitis. Although Ga-67 has been replaced by labeled leukocyte imaging for evaluation of osteomyelitis in most regions of the skeleton, it remains the radionuclide procedure of choice for diagnosing spinal osteomyelitis. Ga-67 frequently is performed in conjunction with bone scintigraphy, and over the years, criteria have been established for the interpretation of bone/Ga-67 imaging. These criteria are used regardless of the area of the skeleton being evaluated (14). The combined test is as follows:
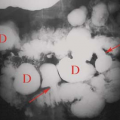
Positive for osteomyelitis when the distribution of the two tracers is spatially incongruent or when the distribution is spatially congruent and the relative intensity of uptake of Ga-67 is greater than that of the bone agent (Fig. 60.7).
Equivocal for osteomyelitis when the distribution of the two radiotracers is congruent, both spatially and in terms of intensity (Fig. 60.8).
Negative for osteomyelitis when the Ga-67 images are normal, regardless of the bone scan findings or when the distribution of the two tracers is spatially congruent and the relative intensity of uptake of Ga-67 is less than that of the bone agent (Fig. 60.9).
Radiolabeled Leukocytes
Although a variety of in vitro leukocyte labeling techniques have been investigated, the only approved methods in the United States employ the lipophilic compounds 111In oxyquinoline and 99mTc-exametazime. The labeling procedure takes about 2 to 3 hours. Approximately 40 to 60 mL of whole blood is withdrawn from the patient into an anticoagulant-containing syringe. All of the cellular components of the blood can be labeled and, therefore, the leukocytes must be separated from erythrocytes and platelets. After withdrawal, the syringe containing the blood is maintained in an upright position for about 1 to 2 hours to promote erythrocyte sedimentation, a process facilitated by the addition of hydroxyethyl starch. This process can be accelerated by substituting
hypotonic lysis of the red cells for gravity sedimentation. After being separated from the erythrocytes, the leukocytes are separated from platelets through centrifugation, and the leukocyte “pellet” that forms at the bottom of the tube is incubated with the radiolabel, washed, and reinjected into the patient (2,15).
hypotonic lysis of the red cells for gravity sedimentation. After being separated from the erythrocytes, the leukocytes are separated from platelets through centrifugation, and the leukocyte “pellet” that forms at the bottom of the tube is incubated with the radiolabel, washed, and reinjected into the patient (2,15).
111Indium-Labeled Leukocytes. The imaging characteristics of 111In, which has photopeaks of 173 keV and 247 keV, are superior to those of Ga-67. This radionuclide decays by electron capture with a half-life of 67 hours. The energies require the use of a medium-energy collimator and a gamma camera capable of imaging multiple energy peaks. Energy discrimination is accomplished by using a 15% window centered on the 174-keV photopeak and a 20% window centered on the 247-keV photopeak of 111In. High target (infection) to background ratios provide excellent image contrast. The spleen is the critical organ, receiving up to 20 rads per mCi of injected cells. As a result, the adult dose of 111In-labeled leukocytes is limited to about 18.5 MBq (500 μCi). Images obtained shortly after injection are characterized by intense pulmonary activity. This activity, which clears rapidly, probably is the result of leukocyte activation during the labeling process, which impedes movement through the pulmonary vascular bed, prolonging their passage through the lungs. At 24 hours after injection, the usual imaging time for In-WBCs, the normal distribution of activity is limited to the liver, spleen, and bone marrow (15).
Advantages of the 111In label are its stability and a virtually constant normal distribution of activity limited to the liver, spleen, and bone marrow. The 67-hour physical half-life of 111In permits delayed imaging, which is particularly valuable for musculoskeletal infection. There is another advantage to the use of In-WBCs in musculoskeletal infection. Patients undergoing labeled leukocyte imaging often require bone or marrow scintigraphy, which can be performed while the patient’s cells are being labeled or as simultaneous dual isotope acquisitions, or immediately after completion of the In-WBC study. If Tc-WBCs are used, an interval of least 48 and preferably, 72, hours is required between the white cell and the bone or marrow scans (15).
Disadvantages of the indium label include a low-photon flux, less than ideal photon energies, and the fact that a 24-hour interval between injection and imaging generally is required (15).
99mTechnetium-Labeled Leukocytes. For Tc-WBC studies, a high-resolution, low-energy parallel hole collimator is used with a 15% to 20% window centered on the 140-keV photopeak of 99mTc. The usual adult dose of Tc-WBCs is 185 to 370 MBq (5 to 10 mCi). The normal biodistribution of Tc-WBCs is more variable than that of In-WBCs. In addition to the reticuloendothelial system, and pulmonary activity soon after injection, activity normally is present in the urinary tract, colon (within 4 hours after injection), blood pool, and occasionally, the gall bladder (Fig. 60.10). The time interval between injection of Tc-WBCs and imaging varies with the indication; imaging usually is performed within a few hours after injection (15).
Advantages of 99mTc-WBCs include a photon energy that is optimal for imaging using current instrumentation, a high-photon flux, and the ability to detect abnormalities within a few hours after injection. Disadvantages include urinary tract activity, which appears shortly after injection, and colonic activity, which appears by 4 hours after injection. The instability of the label and the 6-hour half-life of 99mTc are disadvantages when delayed 24-hour imaging is needed. This occurs in those infections that tend to be indolent in nature and for which several hours may be necessary for accumulation of a sufficient quantity of labeled leukocytes to be successfully imaged (15).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree