Introduction
Monochorionic twin gestations are at increased risk of perinatal morbidity and mortality related to a shared placental circulation. They are uniquely susceptible to the development of twin-twin transfusion syndrome (TTTS), with an incidence of approximately 10% to 15%. TTTS involves unbalanced shunting of blood between twins across vascular anastomoses within the placental bed. Without intervention, early-onset TTTS has a mortality rate that can approach 95%; among survivors, there is a substantial risk for profound neurologic handicap.
Fetoscopic selective laser photocoagulation (SLP) of anastomotic vessels has emerged as the most efficacious therapy to treat TTTS. SLP refers to intentional, laser-induced interruption of pathologic vascular communications linking donor and recipient circulations across a shared placental surface. By interrupting flow through these aberrant channels, the procedure directly disrupts a key component of the underlying disease process. In contrast, amnioreduction is an alternative therapy for TTTS that temporizes the condition indirectly by removing excess recipient twin amniotic fluid. Because amnioreduction addresses a consequence (polyhydramnios) of the disease state, but does not disrupt the underlying disease pathway itself, serial interventions are often required to support an affected pregnancy to an advanced gestational age.
SLP for treatment of TTTS involving laparotomy and direct uterine entry was first described by De Lia et al. in 1990. Advances in technique and technology since that first description permit minimally invasive percutaneous approaches. The procedure introduces custom-designed fetoscopic equipment with articulating eyepieces and fiber optic video displays under continuous ultrasound (US) guidance through percutaneous uterine access. Previously, the procedure involved nonselective photocoagulation of any vessels that crossed the division created by the insertion of the amniotic membrane onto the chorionic plate; however, at the present time, careful evaluation of the vascular equator between the twins targets photocoagulation of identified pathologic anastomoses. Available outcomes data suggest SLP is superior to amnioreduction with respect to survival, and survival free of severe neurologic injury, validating SLP as the optimal therapy for early-onset, severe TTTS ( Chapter 169 ).
Procedure
Description, Technique, and Equipment
In our institution, after providing informed consent, the patient undergoes SLP in a sterile operating room, following administration of preoperative antibiotic prophylaxis. Essential personnel include the operator and assistants, anesthesiologist, surgical technician, circulating nurse, and an assistant to record location and number of placental anastomoses. Evidence suggests that a learning curve exists for achieving SLP proficiency and that the physician performing the procedure should have prior pedagogic and simulation training, with initial procedures supervised by an experienced surgeon.
Although SLP can be performed under anesthesia ranging from local to general, in most centers in the United States, including ours, regional anesthesia is preferred. The anesthetized patient is positioned on the operating table in dorsal position with a slight leftward tilt. After the patient’s abdomen has been prepared and draped, transabdominal US confirms placental location, donor and recipient position, course of the dividing membrane, and placental cord insertion sites for each twin. The axis of the vascular equator is estimated, and an appropriate entry site into the polyhydramniotic recipient sac is determined relative to this estimate. A 5-mm skin incision is made on the maternal abdomen overlying the path of intended uterine entry.
Under continuous US guidance, a 10-F (for 0-degree fetoscope) or 12-F (for 30-degree fetoscope) flexible plastic cannula (Cook Medical, Inc., Bloomington, IN) ( Fig. 115.1 ) loaded with a sharp trocar, is introduced into the recipient sac. The trocar is removed from the cannula, and an amniotic fluid sample is obtained for karyotype analysis (if not previously performed) and other analyses as indicated. At this time, a fetoscope ( Fig. 115.2 ) housed within a paired double-lumen operative sheath ( Fig. 115.3 ), is introduced through the cannula into the polyhydramniotic space. Although fetoscope technical specifications vary, it is important to consider the viewing angle when selecting the proper fetoscope for a given procedure. Although 0-degree fetoscopes are generally used for cases with a straightforward posterior placental approach, in cases with anterior placentation or otherwise challenging access, 30-degree fetoscopy may facilitate adequate visualization ( Fig. 115.4 ). Fetoscopes are attached to light and video output sources ( Fig. 115.5 ), and the use of an articulating eyepiece with the 0-degree fetoscope allows the operator enhanced maneuverability.

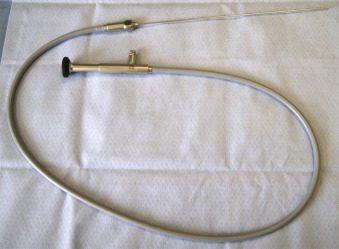
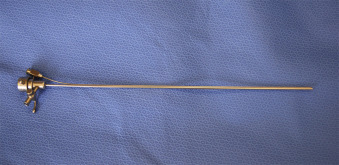


The operative sheath has a large central lumen that accommodates the fetoscope and irrigation through an accessory side port ( Fig. 115.6 ). It also contains a second lumen through which a laser fiber (outer diameter ≤1 mm) can be advanced ( Fig. 115.7 ). Laser sources for SLP include diode, neodymium:yttrium-aluminum-garnet, and potassium-titanyl-phosphate, all selected because their energy absorption profiles fall within the spectrum of hemoglobin. At our center, we use a Medilas D Multibeam (Dornier MedTech, Kennesaw, GA) diode laser device for SLP set between 40 and 60 W.
Under fetoscopic visualization, both twins are identified, and their umbilical cords are traced to their respective placental insertion sites using both fetoscopy and intraoperative US ( and ). The fetoscope allows the team to trace vessels along the chorionic plate, map the vascular equator, and identify pathologic anastomoses. The operator advances the laser fiber for selective photocoagulation of all identified anastomoses, taking care to avoid inadvertent microseptostomy, which can result from photocoagulation through the dividing membrane. Although all visible anastomoses are ultimately targeted, the order in which they are photocoagulated may also be important ( ). Quintero et al. suggested that sequential selective treatment, beginning with photocoagulation of arteriovenous connections (first donor-to-recipient communications, then recipient-to-donor) before arterioarterial and venovenous communications, may provide significant benefit. Further research is needed to determine whether sequential selective photocoagulation strategies correlate with improved procedural outcomes. After all anastomoses have been photocoagulated, a final sweep is conducted to confirm that no vessels have been missed or indicate residual flow.
Fetoscopic SLP of the vascular equator (the Solomon technique) before completion of laser therapy has been recommended to address small anastomoses that might be unaddressed with selective photocoagulation alone. A recent open-label randomized controlled trial has demonstrated that the Solomon technique can reduce complications of recurrent TTTS and twin anemia polycythemia sequence (TAPS), with reduced rates of residual placental anastomoses. When technically possible, the Solomon technique is therefore a reasonable adjunct to SLP.
After photocoagulation, an amnioreduction is performed under continuous US guidance, using the access port of the flexible cannula, to achieve a maximum vertical pocket of 6 cm or less within the recipient sac. The cannula is removed under US guidance. Observation after the procedure includes several hours of continuous external tocometry monitoring. A limited course of perioperative tocolysis may be considered.
Indications
SLP is generally considered for early-onset severe TTTS manifesting between 16 weeks’ and 26 weeks’ gestation. Although international practices vary, in the United States, candidates referred to a fetal care center with trained operators are offered SLP on confirmation of Quintero stage II through IV disease. In some centers, echocardiographic evidence of recipient twin-acquired cardiac dysfunction is considered sufficient to “upstage” TTTS using modified scoring systems, potentially indicating SLP for selected cases with stage I disease.
SLP for stage I TTTS is a controversial practice. Stage I disease has an overall favorable prognosis. In one study by Quintero et al., there was 100% survival of at least one twin without laser therapy in cases of stage I TTTS. High rates of nonprogression or resolution with stage I disease must also be weighed against the risks of any invasive therapy. Retrospective data do not clearly indicate differences in outcomes between SLP and amnioreduction for treatment of stage I disease. However, the available data on stage I outcomes after therapy are limited, conflicting, and potentially biased because these cases are underrepresented in treatment trials.
In an uncontrolled retrospective study reported by Quintero et al. assessing stage-based treatment, an inverse relationship was observed between survival and stage in the group undergoing serial amnioreduction. This same trend was not seen in the SLP group, where outcomes were relatively uniform across all stages. When modalities were compared, benefit of SLP was apparent only with advanced stage disease. Based on these data, the authors recommended offering serial amnioreduction for stage I disease, offering either amnioreduction or SLP for stage II disease based on a consideration of gestational age, and reserving SLP for stage III and IV disease. In contrast, Huber et al. reported overall improved, stage-based outcomes within a prospective cohort undergoing SLP, despite a trend toward worsening outcomes with advancing stage. Although this series lacked an amnioreduction comparison group, based on the favorable outcomes, the authors concluded that SLP should be offered across all TTTS disease stages but that amnioreduction may be considered with stage I disease manifesting at an advanced gestational age.
To date, there are no results from a well-designed prospective trial designed to compare outcomes for stage I disease with SLP compared with amnioreduction or expectancy with close observation for progression of disease. Until such time, SLP for stage I disease remains a matter for debate. In most U.S. fetal therapy centers, contemporary practice includes expectant observation after a diagnosis of early-onset stage I TTTS. Although it might be tempting to proceed with amnioreduction in such a case, a complication such as preterm premature rupture of membranes (PPROM), bleeding, or amnion-chorion separation may preclude treatment with SLP should the disease progress to a more advanced stage.
Contraindications
Absolute contraindications to SLP include PPROM, preterm labor, chorioamnionitis, active abruption, and a maternal bleeding disorder or medical condition that would preclude percutaneous uterine access. Relative contraindications include major fetal anomalies, aneuploidy, short cervix, amnion-chorion separation, and maternal human immunodeficiency virus or hepatitis C infection.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






