Kamran Shaikh, MD, FASE, FACC
Dipan J. Shah, MD
INTRODUCTION TO ASSESSMENT OF SEMILUNAR VALVES BY CARDIOVASCULAR MAGNETIC RESONANCE
The assessment of valvular heart disease involves:
• assessment of the structure and motion of the valve, typically with steady-state free precision (SSFP) and gradient-echo cine techniques.
• assessment of the severity of valvular stenosis of regurgitation, typically with in-plane and through-plane velocity-encoded (VENC) cine acquisitions.
• assessment of lesions on the valve and subvalve apparatus typically with T1-weighted fast-spin echo and postcontrast sequences when echocardiography is inadequate.
Traditional gradient-echo cine is very useful in situations where there is flow artifact or metallic artifact in the SSFP images and for identification of flow turbulence from stenotic or regurgitant valves.
The assessment of mechanism of aortic and pulmonic valve stenosis involves acquiring high-resolution SSFP cines of the valve. Since there is a free choice of imaging planes by cardiovascular magnetic resonance (CMR), the operator can ensure that valve cines are acquired at the tips of the valve. For the aortic valve, 3-chamber (3 CH) and left ventricular outflow tract (LVOT) cines can be used since their imaging planes are perpendicular to each other and both give excellent longitudinal views of the aortic valve. For the pulmonic valve, right ventricular (RV), 3-CH and RV outflow tract (RVOT) views can be used to acquire high-resolution longitudinal cines of the pulmonic valve. Using at least 2 longitudinal cines as a reference point, the operator can prescribe high-resolution cross-sectional cines of the valves at the leaflet tips. These images can be used to planimeter the anatomic valve area in peak systole.
The assessment of severity of aortic and pulmonic valve stenosis involves acquiring phase-contrast (PC) VENC cines. For the aortic valve, in-plane 3-CH and LVOT PC cines are acquired to see the location of flow turbulence at the aortic valve, then through-plane PC cines are acquired perpendicular to the region of maximal flow velocity. The through-plane cines are acquired with progressively higher VENC setting until flow aliasing is no longer present. In our experience this method allows the best determination of peak velocity (PV). An excessively high VENC insures avoidance of aliasing but also introduces errors due to limited dynamic range and also should be avoided. For the pulmonic valve, similar in-plane and through-plane sequences are acquired across the pulmonic valve to assess severity.
The assessment of mechanism of aortic and pulmonic valve insufficiency involves acquiring standard SSFP cines and high-resolution SSFP sequences of the standard echocardiographic (ECHO) views of the heart. The reverse flow in diastole from aortic valve insufficiency (AI) and pulmonic valve insufficiency (PI) cause signal-void in the LVOT and RVOT respectively. The direction of the signal-void and the structure of the valves can provide insight into the mechanism of insufficiency.
The assessment of severity of the aortic and pulmonic valve insufficiency involves acquiring through-plane PC cines at the aortic root and proximal pulmonary artery trunk. Then one can analyze the flow on an off-line workstation to assess for the forward and reverse flow (shown in case studies).
AORTIC VALVE INSUFFICIENCY CASE STUDIES
CASE 1 Aortic Valve Insufficiency in a Native Aortic Valve
A 21-year-old man presented with shortness of breath. He had a murmur since the age of 5 years. He had undergone yearly ECHOs since childhood, which suggested mild AI and aortic stenosis (AS). Three months prior to CMR, he developed progressively worsening shortness of breath. A transthoracic echocardiogram (TTE) suggested at least moderate AI and mild AS. CMR was ordered to further quantify the severity of the AI.
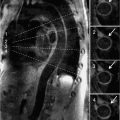
The CMR images are shown in Figures 7B-1 through 7B-3 and Videos 7B-1 through 7B-3. Figure 7B-1 is a series of SSFP images. Figures 7B-1A and 7B-1B show the diastolic and systolic frame from a mid-LV short-axis acquisition which shows a dilated LV; quantitation yielded an end-diastolic volume (LVEDV) of 212 mL, end-systolic volume (LVESV) of 58 mL, end-diastolic dimension (LVEDD) of 6.0 cm, and end-systolic dimension (LVESD) of 3.3 cm. Figure 7B-1C is the diastolic frame of the 3-CH view which shows the eccentric jet of AI.Figure 7B-1D is a high-resolution diastolic frame of the aortic valve showing the AI. Figure 7B-2 is a series of PC images.Figures 7B-2A and 7B-2B are diastolic frames of in-plane VENC cines showing the AI in the LVOT. Figures 7B-2C and 7B-2D are diastolic frames of through-plane VENC cines at a higher VENC setting showing the AI. A higher VENC was needed because the patient also has moderate AS. Figure 7B-3 shows the offline analysis of through-plane VENC cine across the aortic root. Based on a reverse volume of 75 mL and forward volume of 150 mL, the regurgitant fraction (RF) was calculated as 50% consistent with severe AI. He also had moderate AS with a planimetered valve area of 1.4 cm2. The patient subsequently underwent aortic valve replacement with a St. Jude mechanical valve.
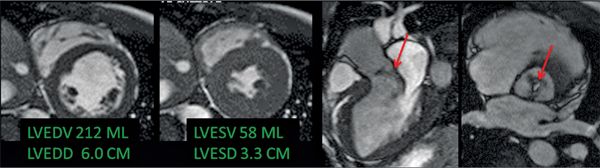
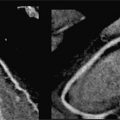
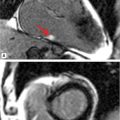
FIGURE 7B-1 End-diastolic and end-systolic frames (left 2 panels) from a mid-short-axis plane show a dilated LV. Quantitative results are shown, indicating LV dilatation by 1D and volumetric analyses. SSFP imaging in 3-CH and aortic valve en face planes (right 2 panels) shows signal-void due to the turbulent flow (red arrows) of aortic regurgitation.
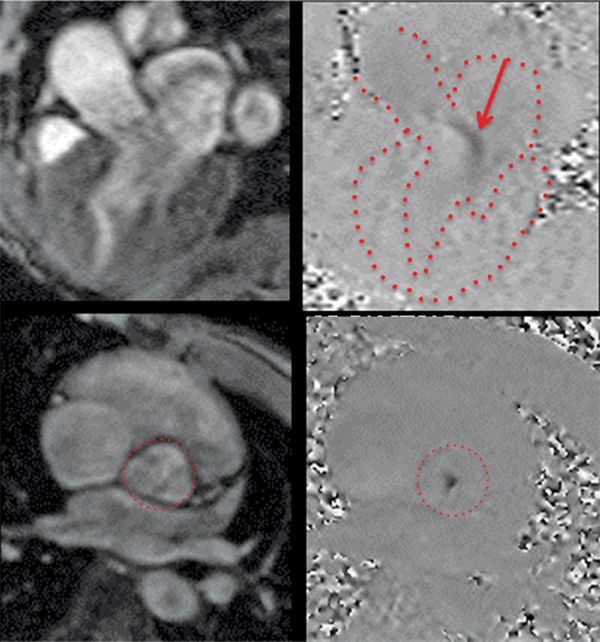
FIGURE 7B-2 Magnitude (left panels) and PC (right panels) images from 3 CH in-plane (top row) and AV through-plane (bottom row) VENC cine acquisitions show the aortic regurgitation. The LA, LV, aortic root, and aortic valve are outlined.
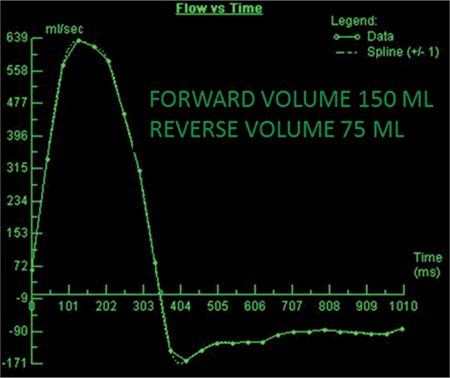
FIGURE 7B-3 Quantification of through-plane VENC cine acquisition yields a forward volume of 150 mL and reverse volume of 75 mL across the aortic valve.
CASE 2 Aortic Valve Insufficiency in a Patient With Marfan Sydrome
A 28-year-old man with no prior medical history presented with new-onset dyspnea. He denied cigarette smoking or alcohol use. He was not on any medication at the time of admission to the hospital. There was no family history of sudden cardiac death. A month prior to admission he was running 3 miles daily. On physical examination, the height was 190.5 cm and the weight was 73.9 kg consistent with a tall, thin habitus. Vital signs on admission were stable. Cardiac examination revealed a grade III/VI early diastolic murmur. The patient underwent an ECHO that showed a dilated LV, moderate to severe aortic valve insufficiency and dilated aortic root. CMR was ordered to assess for the severity of aortic valve insufficiency and to more thoroughly assess the aorta in anticipating possible surgery.
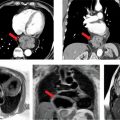
CMR images are shown in Figures 7B-4 through 7B-6. Figure 7B-4 is a series of SSFP images. Figures 7B-4A and 7B-4B show the diastolic and systolic frame of the mid-LV short-axis indicating a moderately enlarged LV. Figure 7B-4C is the diastolic frame of the LVOT view, which shows the eccentric jet of AI and severely dilated aortic root, also seen in the 3-CH view (Figure 7B-4D). Figures 7B-4E and 7B-4F are high-resolution (small field-of-view) diastolic and systolic frames, respectively, showing the AI jet in diastole and normally-opening trileaflet aortic valve in systole. AI is even more evident by through-plane VENC cine acquisition at the level of the aortic valve (Figure 7B-5). Figure 7B-6 shows volume rendering of the magnetic resonance angiography (MRA) of the ascending aorta with a severely dilated aortic root and ascending aorta, maximum dimension 9.2 cm.
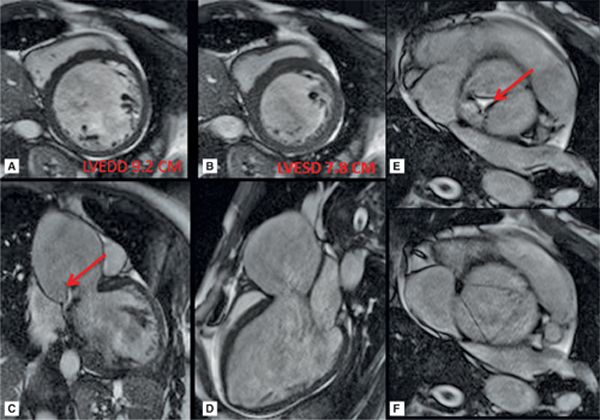
FIGURE 7B-4 SSFP images in short-axis and ED and ES (A and B) show a moderately enlarged LV, with LVEDD of 9.2 cm and LVESD of 7.8 cm. Diastolic frame in the LVOT (C) shows a jet of aortic regurgitation (red arrow) as well as a markedly dilated aortic root, also seen in the 3-CH view (D). Diastolic (E) and systolic (F) frames from a small field-of-view, high-resolution cine show the trileaflet aortic valve en face with AI jet appearing bright (red arrow, E).

FIGURE 7B-5 Magnitude (left panel) and PC (middle panel) images from an aortic valve through-plane VENC cine acquisition show the aortic regurgitation jet (red arrow) in this patient with Marfan syndrome. Quantitation (right panel) demonstrated severe AI. As with Doppler ECHO the abrupt termination of regurgitant flow indicates significantly elevated LV filling pressure.
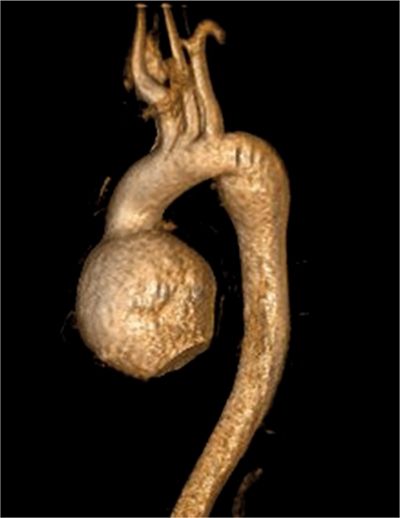
FIGURE 7B-6 Volume rendering of the MRA shows severe dilatation of the aortic root and ascending aorta in a patient with Marfan syndrome; maximum dimension was 9.2 cm.
The patient underwent surgical resection of the aortic valve, aortic root, and ascending aorta. A 31 mm St. Jude conduit valve was used for the aortic valve replacement. The coronary arteries were reimplanted onto the conduit. The patient did well during the postoperative course and was discharged home in stable condition.
CASE 3 Paravalvular Aortic Valve Insufficiency in a Patient With Mechanical Aortic Prosthesis
A 60-year-old man presented with progressively worsening dyspnea. His past medical history included aortic stenosis for which he had undergone aortic valve replacement with prosthetic aortic valve 6 years prior to the CMR study. He had a TTE and transesophageal echocardiogram (TEE) which showed moderate paravalvular AI. CMR was ordered to further characterize the severity and location of paravalvular AI.
CMR images are shown in Figures 7B-7 through 7B-9, and Videos 7B-4 and 7B-5. Figure 7B-7 and Video 7B-4 are a series of SSFP and gradient-echo acquisitions. Figures 7B-7A and 7B-7B show the diastolic and systolic frames of the mid-LV short-axis view which show moderately enlarged LV with mild concentric left ventricular hypertrophy (LVH). Figure 7B-7C is the diastolic frame of the LVOT view which shows the paravalvular AI. Figure 7B-7D is a high-resolution gradient-echo acquisition showing the anteriorly located paravalvular AI. Figure 7B-8, and Videos 7B-5A and B and 7B-5C and D are in-plane and through-plane VENC cine acquisitions, respectively, showing the AI jet. Figure 7B-9 shows the offline analysis of the flow in the aortic root that indicates that the paravalvular AI is severe (regurgitant volume [RV] 72 mL; RF 57%).
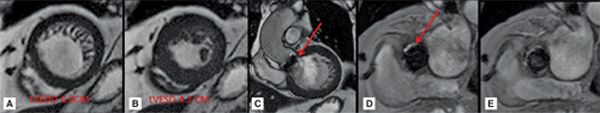
FIGURE 7B-7 End-diastolic (A) and end-systolic (B) frames from a short-axis SSFP cine acquisition show moderate enlargement of the LV with mild concentric LVH. A diastolic frame from an LVOT SSFP acquisition shows the paravalvular AI (C, arrow). The jet is further localized to the anterior paravalvular region by en face gradient-echo imaging, evident as a jet in diastole (D, arrow) not present during systole (E).
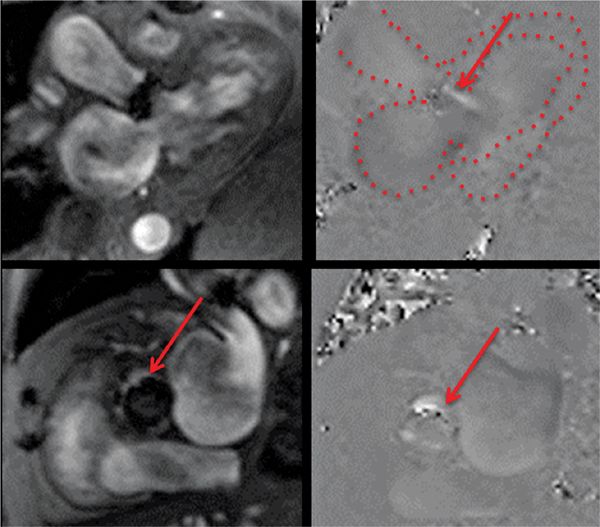
FIGURE 7B-8 Magnitude (left panels) and PC (right panels) reconstructions from in-plane 3CH (top panels) and through-plane aortic valve (bottom panels) VENC cines show a jet of paravalvular AI (red arrows). In the upper right panel, the LA, LV and aortic root are outlined.
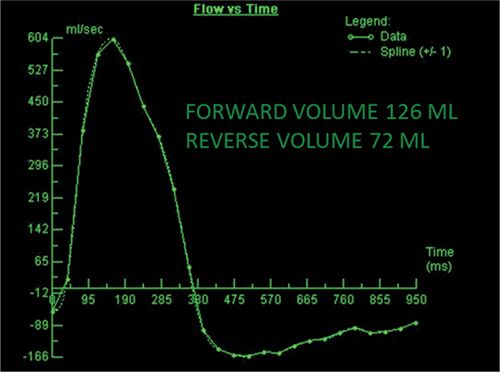
FIGURE 7B-9 Quantification of through-plane VENC cine data yields a forward volume of 126 mL and reverse volume of 72 mL, or RF of 57% consistent with severe AI.
Based on CMR findings of severe paravalvular AI, this symptomatic patient underwent redo aortic valve replacement.
CASE 4 Aortic Valve Insufficiency in a Patient With a Supracristal Ventricular Septal Defect
A 51-year-old Hispanic man presented with shortness of breath. He had no prior medical history and had not seen a physician in the past. He underwent TTE and TEE as part of his work up. Both studies demonstrated severe AI and a ventricular septal defect (VSD). Both studies, however, were inconclusive for the location of VSD and could not reliably quantify shunt fraction. CMR was ordered to better characterize VSD location, to calculate shunt fraction (Qp/Qs), and to assess for any other congenital anomalies.
CMR images are shown in Figures 7B-10 through 7B-13. Figure 7B-10 is a series of SSFP images. The first 2 panels in Figure 7B-10 show the diastolic and systolic frames of the mid-LV short-axis which show moderately enlarged LV. The third panel in Figure 7B-10 is the diastolic frame of the 3-CH view which shows the AI. In Figure 7B-11 gradient-echo cine images acquired in a basal short-axis view at the level of the aortic valve indicate the AI in diastole due to poor coaptation of right coronary cusp and VSD jet in systole. Figure 7B-12 shows images of a 3-CH VENC cine acquisition with an eccentric jet of AI along the anterior mitral leaflet (the basis for the Austin-Flint murmur, where a mitral stenosis rumble is produced by significant AI rather than mitral valve disease). Figure 7B-13 shows the off-line analysis of the flow in the proximal ascending aorta that indicated that AI was severe (RV 74 mL, RF 58%).
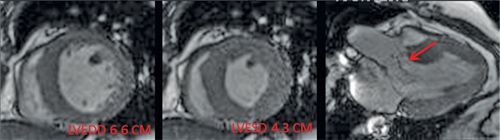
FIGURE 7B-10 SSFP images show dilatation of the LV and signal-void due to AI (red arrow).
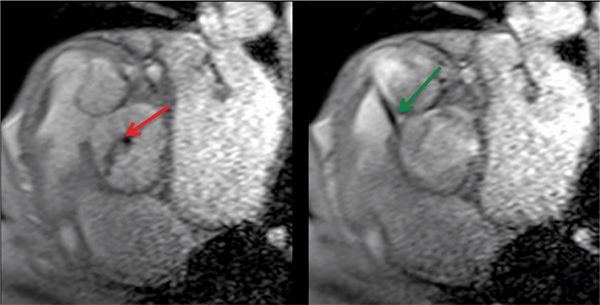
FIGURE 7B-11 Gradient-echo diastolic (left) and systolic (right) frames show AI (red arrow) and a VSD jet (green arrow) into the RVOT.
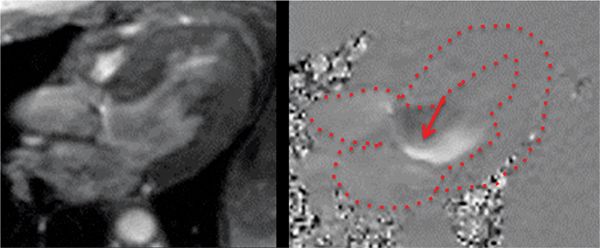
FIGURE 7B-12 In-plane VENC cine in a 3-CH view with magnitude (left) and PC (right) images in diastole shows the eccentric jet of AI. Note that the jet travels along the anterior mitral leaflet.
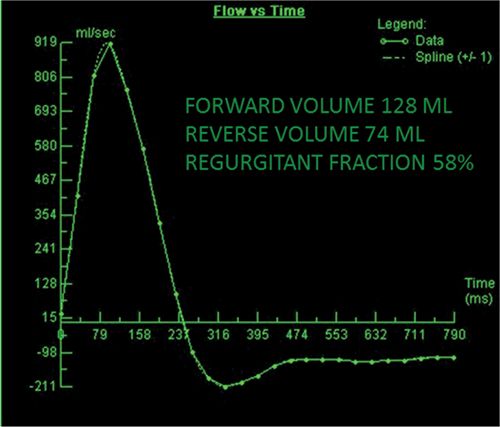
FIGURE 7B-13 Quantification of VENC cine data revealed severe AI, with a RF of 58%.
The net systemic flow was 3.9 L/min (Qs) and the net pulmonary artery flow was 5.4 L/min. The shunt fraction (Qp/Qs) was 1.4:1 with net shunt flow of 1.5 L/min.
Supracristal VSD is a very rare form of ventricular septal defect (<1%). The VSD is located in the LVOT underneath the right coronary cusp and communicates with the RVOT. Due to Venturi effect from the VSD flow, the right coronary cusp of the aortic valve does not properly coapt with the other leaflets resulting in AI.
DISCUSSION: AORTIC INSUFFICIENCY
The etiologies of AI include congenital (bicuspid aortic valve, supracristal VSD), genetic (Marfan syndrome, Ehler-Danlos sydrome), calcific degeneration, rheumatic, systemic hypertension, acute dissection, and rheumatological (rheumatoid arthritis, ankylosing spondylitis). The presentation with AI may be acute as in acute Type A aortic dissection or chronic with progressively worsening cardiovascular symptoms.
Current societal guidelines recommend ECHO as the imaging modality of choice to diagnose AI, assess the severity of AI, assess aortic root size, and follow-up patients with AI.1 CMR is recommended as the initial and follow-up test for assessment of LV volumes and function in patients with suboptimal acoustic window. CMR-based AI severity assessment relies on through-plane VENC cine acquisition, with grading by RF as mild (RF <15%), moderate (RF 16%-25%), moderate to severe (RF 26%-48%) or severe (RF >48%).2 The comprehensive CMR examination in a patient with aortopathy and aortic valve disease encompasses assessment of LV volumes and an MRA; it is, therefore, an excellent tool to serially assess the patient with AI for progressive dilation or disruption of the aorta and for LV enlargement. In the asymptomatic patient with AI, LV enlargement alone may warrant valve replacement or repair. Results from a large prospective study done in patients with moderate or severe AI supports serial CMR as a better strategy than serial TTE to predict subsequent need for surgery.3
AORTIC VALVE STENOSIS
CASE 5 Aortic Stenosis in a Native Aortic Valve
A 69-year-old woman presented with progressive shortness of breath and an episode of syncope. She had a history of hypertension and rheumatoid arthritis. She had undergone TTE that demonstrated severe degenerative aortic stenosis (aortic valve area [AVA] 0. 8 cm2 and mean gradient 40 mm Hg), but the aortic valve and aortic root were not well visualized because of poor acoustic window and severe calcification of the aortic valve. CMR was considered as a noninvasive option since the patient could not undergo TEE because of esophageal stricture.
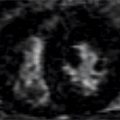
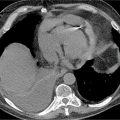
CMR was performed and the images are shown in Figures 7B-14 through 7B-16. Figure 7B-14 is an SSFP frame in the 3-CH view showing severe flow acceleration across the aortic valve and turbulence in the aortic root. Figure 7B-15 shows a high-resolution SSFP systolic frame of the aortic valve, which allows for direct planimetry of the aortic valve; aortic stenosis was deemed severe based on a planimetered valve area of 0.9 cm2. Figure 7B-16 shows through-plane VENC imaging, first at a VENC of 400 cm/s and then with a higher VENC of 425 cm/s to eliminate aliasing. Thus, the PV is between 400 and 425 cm/s. Due to slight underestimation of PV by CMR due to partial volume effects (from both temporal and spatial averaging), we typically will round the PV up to the higher valve in the range. Using the simplified Bernouli equation this would translate to a PG across the aortic valve of approximately 72 mm Hg.
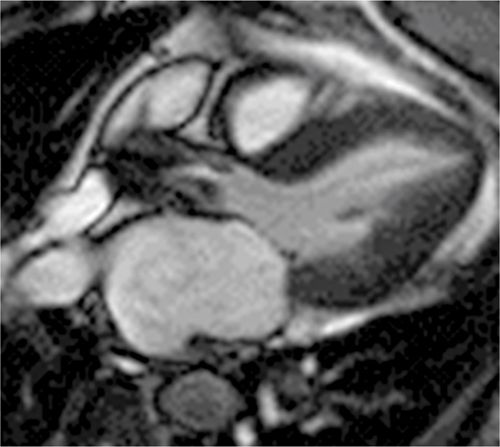
FIGURE 7B-14 SSFP frame from a 3-CH cine acquisition shows severe flow acceleration across the aortic valve and turbulence in the aortic root.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree