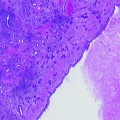
Fig. 27.1
The presence or absence of signs of cellular damage may not necessarily equate to cellular viability, particularly in the sublethal border zone just beyond the “kill-zone”
Post-radiotherapy tissue changes are unique from that of other ablative modalities, evolving over several years as postmitotic cell death occurs due to long tumor doubling times [29]. Bostwick et al. described that nonneoplastic irradiated glands often demonstrated atrophy, squamous metaplasia, and moderate to severe cytologic atypia [30]. However, both benign and neoplastic glands displayed cytologic abnormalities including variable nuclear size and shape, abnormal chromatin staining, and prominent nucleoli. Brawer et al. evaluated 37 postirradiation biopsy specimens, demonstrating an unequivocal diagnosis of persistent cancer in 62%, no evidence of residual carcinoma in 21.6%, and an uncertain diagnosis due to severe radiation damage in 11% [31]. In the latter group, observations included glands lined by piled up atypical pleomorphic cells without definitive evidence of stromal invasion, gland within gland proliferation, or basal cell layer loss. Positive basal cell specific staining of keratin (KA1) enabled the authors to distinguish benign radiation-induced atypia from persistent cancer which failed to stain for KA1.
Given that some patients with negative posttreatment biopsies go on to locally recur [32], whatever histopathologic methods are used, the accurate determination of a true negative biopsy is of paramount importance.
PSA and Definition of Biochemical Failure After Focal Ablation
No standard definition for treatment failure has been established following FA in part due to the variety of treatment strategies that fall under this broad classification. Some authors have used the American Society for Therapeutic Radiology and Oncology (ASTRO) definition of biochemical failure (three consecutive rises in PSA), whereas others have relied on the Phoenix/Houston definition (PSA nadir + 2 ng/mL) (Table 27.1). Unlike whole gland treatment strategies, subtotal treatments preserve a higher volume of PSA producing tissue, obfuscating conventional definitions of failure [41]. Thus, the PSA nadir may not accurately reflect biochemical disease-free status in the setting of untreated viable tissue. Following focal cryoablation, Lambert et al. considered several PSA thresholds to define biochemical recurrence [37]. At a PSA nadir less than 1.0 ng/mL, 64% of the patients biochemically failed, but none had biopsy-proven cancer. Based on an analysis of a cohort of 25 patients, the authors recommended a new definition of biochemical recurrence for focal cryoablation, based on a modified Phoenix definition: a PSA nadir of greater than 50% of the pretreatment PSA level or a PSA nadir plus 2 ng/mL; however, posttreatment biopsies were only obtained for 28% of patients, follow-up was short at a median of 28 months, and no subsequent external validation was completed. On the other hand, Lindner et al. found no correlation between the presence of residual or new disease on post-ablation biopsy and nadir PSA, time to nadir PSA, or PSA velocity following interstitial photothermal focal therapy [42]. Other definitions which have been applied to whole-gland ablation, such as the “Stuttgart definition” of PSA nadir + 1.2 ng/mL, have yet to be tested in FA series [43]. The value of ancillary PSA data such as PSA velocity and slope, free vs. total PSA, PSA doubling time, and PSA density (PSA divided/remaining volume of glandular tissue) has not been established yet.
Table 27.1
Prostate-specific antigen and definition of biochemical failure after focal ablation
Modality and tumor criteria | Focal Ablation Scheme | Biopsy | PSA | Functional FU | Failure definition | Imaging | |
|---|---|---|---|---|---|---|---|
Ahmed [33] (n = 20) | HIFU, Gleason 4 + 3 or less, unilateral disease T2b or less, PSA 15 ng/mL or less | Hemiablation | @ 6 months biopsy of treated side sampling every 1 mL tissue with at least 1 core; contralateral biopsy of suspicious lesions on mp-MRI | q3 months | IPSS, IIEF-15,UCLA-EPIC, FACT-P @ 12 months | Primary outcome was potency | mp-MRI with gadolinium at 1 month and 6 months |
Lindner [34] (n = 12) | Photothermal therapy (Indigo Optima laser), Gleason 6 or less, T2a or less, <30% cores+, <50% cancer/core, PSA <10 ng/mL, tumor in 1 sector only | Abnormal lesion on mp-MR | 10-core + 2 AZ @ 3–6 months | PSA nadir, PSA velocity | IPSS, IIEF-5 @ 1, 3, and 6 months | Persistent tumor in treatment area | mp-MRI with gadolinium and CEUS at 7 days |
Muto [35] (n = 29) | HIFU Any Gleason, T1c-T2a, Unilateral cores | Bilateral peripheral zone and ipsilateral transition zone | Sextant @ 6 and 12 months | 3, 6, 12, 18, 24, and 36 months | UCLA-PCI and IPSS, uroflow, Testosterone @ 6 months, 12 months | ASTRO | Preoperative: pelvic MRI Postoperative: none |
Ellis [36] (n = 60) | Cryosurgery Any Gleason, T1c-T2c | Hemiablation | Rising PSA | 6 weeks, then q3 months during first year, then q6 months thereafter | Incontinence (any leakage) and impotence (erection sufficient for intercourse w/ or w/o pharmacologics) assessed via patient interview @ 6 months | ASTRO | None |
Lambert [37] (n = 25) | Cryosurgery Gleason 6–7, T1c, Unifocal disease (1–2 contiguous bx cores, <10% volume of 12 cores) | Hemiablation | 12 core postoperatively | q3, 6, 12, and then q6 months | Physical examination, questionnaires on potency, urinary retention, incontinence, rectal and perineal pain, fistula formation, potency (erection sufficient for penetration) | PSA nadir + 2 ng/mL or PSA nadir of <50% | None |
Onik [38] (n = 21) | Cryosurgery unilateral cancer | Targeted ablation of abnormal Doppler US area | @ 1 year: sampling of treated and untreated lobe, + abnormal areas on color Doppler US, 8 cores from PZ | q3 months × 2 years, then q6 months | Written questionnaire and telephone: erections sufficient for penetration and satisfactory, #pads, any complications | ASTRO | Color Doppler as part of biopsy protocol; MRSI in one patient |
Bahn [39] (n = 21) | Cryosurgery unilateral cancer | Hemiablation | @ 6 months, 1, 2, and 5 years or three consecutive PSA rises | q3 months | Incontinence: any leakage of urine > 3 months Potency: Modified Brief Male Sexual Function Index questionnaire | ASTRO | None |
Dhar [40] (n = 795) | Cryosurgery | NR | Physician discretion | NR | Incontinence: any pad use Potency: penetration with or without assistance | ASTRO and Phoenix | None |
Despite the difficulty of interpreting PSA data in this setting, PSA should be obtained regularly (e.g., every 3 months during year 1, q6 months thereafter). Following successful FA, the PSA should nadir to a nonzero level commensurate with the extent of ablation and remain stable. Indolent progression of BPH or small untreated “insignificant tumors” should not be expected to affect serum PSA appreciably. By contrast, rapid progression of PSA kinetics is worrisome for recurrent disease or progression of existing untreated tumors. With sufficient long-term follow-up (e.g., 15–20 years), recurrence-free, cancer-specific, and overall-survival outcomes will ultimately provide definitive treatment efficacy information.
Post-ablation Imaging
In cases where preoperative imaging identifies a discrete lesion, a posttreatment MRI, PET/CT [44], or CEUS at 1 week may demonstrate a hypoperfusion or photopenic defect [34, 42]. Following a baseline scan to confirm ablation of the target, regular follow-up imaging starting at approximately 6 months (following radiographic stabilization of the treated area) may provide a useful adjunct to biopsy. At the present time, although multiparametric MRI is the most widely used imaging platform, the resolution for detection of small tumors is limited. Emerging molecular imaging technologies utilizing novel radionuclides or radiolabeled prostate specific antibodies may ultimately provide a high degree of sensitivity and specificity.
MRI
In the past 5 years, an increasing number of publications have emerged evaluating the role of MRI of the prostate. The role of MRI has been assessed in a number of settings including diagnosis, staging, operative planning, and posttreatment evaluation [45]. After treatment with cryoablation, HIFU, or PDT, coagulative necrosis of the targeted areas ensues resulting in a characteristic hypointense lesion on T2-weighted (T2W) MRI [46–48]. Similar changes occur in the prostate after radiotherapy including prostate shrinkage and fibrosis, diffuse reduction in T2 signal intensity, and zonal indistinctness which creates problems for T2W image analysis [49].
Anatomic T2-weighted imaging alone has been demonstrated to be insufficient to discriminate tumor recurrence in untreated prostate tissue [50]. The detection rate of standard unenhanced T2-weighted MRI for prostate cancer ranges from 37 to 96%. In a study by Noguiera et al., among a highly selected group of 202 patients with low-risk biopsy characteristics who would be ideal candidates for focal ablation or active surveillance (i.e., Gleason 6, 1 positive core < 2 mm, PSA density ≤ 0.10, and cTstage ≤ T2a) scheduled to undergo radical prostatectomy, a retrospective review compared preoperative T2-weighted MRI against whole-mount RP specimens. Consistent with similar series that have demonstrated understaging of low risk patients, whole mount demonstrated that 81% of patients had multifocal tumors, 68% had bilateral tumors, and only 22% and 51% were pT2a or pT2b, respectively. T2W MRI performed poorly in this subset of patients, correctly predicting minimal disease in only 9% of cases, with an area under the curve (AUC) for predicting tumor extent of just 0.624. Another major limitation of T2WI is its inability to detect small tumors. Roethke et al. correlated pre-prostatectomy T2W endorectal MRI with whole-mount RP pathology [51]. T2W MRI successfully visualized 0%, 3%, 13%, 45%, and 89% of lesions measuring <0.3 cm, 0.3–0.5 cm, <1 cm, 1–2 cm, and >2 cm, respectively. Unfortunately, the independent effect of grade on MRI visualization of tumors was not reported. Ikonen et al. found slightly improved accuracy detecting 5% of tumors <5 mm but 89% of lesions >1.0 cm [52].
T2-weighted MRI, particularly at 1.5 T, has limited ability to detect tumor recurrence in treated tissue, with poor resolution, particularly for small, low grade tumors that focal ablation primarily targets. Several studies have reported low sensitivity and specificity for tumor recurrence in irradiated prostates using T2W MRI [53]. Mp-MRI, however, particularly when used in conjunction with an endorectal coil, provides not only anatomic T2-weighted images, but also functional image sequences [e.g., dynamic contrast-enhancement (DCE) and diffusion-weighted imaging (DWI)] which exploits the different properties of benign and malignant prostate tissue. The combination of these techniques provides a higher degree of accuracy for cancer detection and staging [45, 54–56]. Delongchamps et al. performed mp-MRI with an endorectal coil in 58 consecutive patients with low risk features (Gleason 6, ≤2 contiguous sextants, core involvement ≤7 mm, total length ≤1 cm, <1/3rd of total cores positive, PSA <10 g/mL) undergoing radical prostatectomy [56]. A two-reader consensus blinded to pathologic information was conducted, assigning a score on a 3-point scale to three sequences: T2W, DWI (quantitative ADC mapping), and DCE (K trans, k ep, and initial area under the gadolinium concentration curve @ 60 s). Imaging information was then compared against pathological information. The combination of all three imaging sequences was found to provide the highest receiver operating characteristic curve accuracy (AUC = 0.90) for detection of cancer in the peripheral zone compared with T2W alone (AUC = 0.77), or combinations of other sequences (T2W + DWI = 0.84, T2W + DCE = 0.88). The reported sensitivity and specificity of the combination of three sequences for the detection of tumors >0.2 cm3 was 75% and 94%, respectively. Kim evaluated mp-MRI against biopsy results following whole gland HIFU in 27 patients with a rising serum PSA. Sixty-seven percent of patients were found to have residual tumor (61% of which were HR) [57]. The average AUC for detection of tumor by receiver operating characteristics curve analysis (ROC) was 0.81 for DCE alone and 0.79 for T2WI with DWI. Therefore, mp-MRI at 3 T provides an increased signal-to-noise ratio with reasonable accuracy for the detection of residual or recurrent disease in this setting.
After whole-gland HIFU, cryotherapy, or radiation, on T2W imaging, the prostate becomes diffusely hypointense or heterogenous with loss of zonal anatomy, creating difficulty for detection of tumor recurrence [53, 58]. In a study by Cheikh et al., at a mean of 22.5 months after whole-gland HIFU, 15 patients with suspected tumor recurrence under T2W and DCE 1.5 T MR evaluation followed by a transrectal ultrasound-guided biopsy [58]; the biopsy protocol included sextant biopsies with additional biopsies taken in areas of color Doppler abnormality or MR abnormality. Among 13 of 15 patients who had tumor recurrence on biopsy, T2W alone was difficult to interpret and resulted in a high rate of false negative calls. DCE using FLASH T1W fat suppressed images, on the other hand, demonstrated early enhancement in a number of cases, correlating with positive biopsies more frequently.
Two FA trials have incorporated mp-MRI into their pre- and post-treatment protocols. Ahmed and Emberton performed mp-MRI consisting of T2WI, DCE, and DWI before and after hemiablative HIFU [6]. At 1 month following ablation, a follow-up scan was performed to confirm tumor ablation by demonstrating a confluent lack of enhancement in the treated lobe. Imaging was concordant with posttreatment biopsy information demonstrating treatment success in 18 of 20 patients.
Linder and colleagues used photothermal ablation of lesions that were visible on mp-MR in conjunction with CEUS with microbubbles [42]. Following treatment, MRI and CEUS were repeated at 7 days to confirm hypovascularity in the treated region. The area of hypoperfusion on MR was an average of 12.3 times the pretreatment tumor contour. In a follow-up study, the authors compared the laser ablation volume on contrast-enhanced MRI with the ablation volume on prostatectomy specimen assessed with H&E and cytokeratin 8 (CK8) immunohistochemical stains. Considering a positive CK8 stain as a surrogate for vital or viable tissue, the authors found no evidence of viable cells following laser ablation. In addition, a good correlation between MR-calculated volume and histopathologic ablation volume was found (MR volume = 1.4 × H&E volume, MR volume = 1.1 × CK8 volume). The above studies illustrate the value of postoperative imaging when the tumor was visualized by the same imaging modality before treatment.
MR Spectroscopy
MR spectroscopy (MRSI), like DWI and DCE, provides adjunctive functional information which can be obtained regardless of altered anatomy in treated tissue. In both treated and untreated prostate tissue, MRSI relies on metabolic differences between tissues. Untreated cancerous prostate tissue characteristically displays a biochemical signature with elevated choline (due to high cell membrane turnover) and low citrate levels compared to benign tissue [53, 61]. In the primary setting, the choline-plus-creatine-to-citrate ratio is the main metabolic criterion that is used to identify cancer [62]. Following radiation, citrate and polyamine spectral peaks progressively wane over time, often to undetectable levels, which some have indicated may limit the use of these standard criteria [63]. However, Pucar and colleagues retrospectively correlated MRSI with salvage RP specimens following EBRT in nine patients [64]. The authors graded voxels with diagnostic levels of citrate in which the choline + creatine ratio was ≥0.5 and any voxels with diagnostic levels of choline as suspicious for recurrent tumor in the peripheral zone. ROC curve analysis demonstrated a threshold choline + creatine to citrate ratio of 0.5 achieving the highest AUC (0.875) for the detection of recurrent tumor; in some cases, benign areas were found to be falsely graded as metabolically abnormal. Others have noted that elevated choline levels have been found to correlate with tumor recurrence after radiotherapy or cryotherapy [65]. Coakley et al. [49] reported an AUC of 0.81 for the detection of locally recurrent prostate cancer after radiotherapy using an elevated choline level (1.5:1) relative to creatine compared with an average AUC of 0.50 with standard T2W imaging alone (if creatine was undetectable, a choline peak area-to-noise ratio >5:1 was considered elevated). Following cryotherapy, Parivar et al. found that MRSI significantly increased their ability to detect recurrent tumor compared with T2W MR alone [66]. In areas confirmed pathologically to contain cancer, the average choline + creatine/citrate ratio was 2.4 (±1.0) compared with 0.56 (±0.2) in benign areas. After HIFU treatment, however, the role of MRSI at the present time is less clear [47




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree