Key Points
- ▪
Although echocardiography plus MRI is still the best overall modality for the evaluation of complex congenital heart lesions, especially postoperatively, there are exceptions.
- ▪
As children and adults with congenital disease age, a growing number of them acquire contraindications to CMR, such as pacemakers and ICDs.
- ▪
Many surgical and interventional procedures use metallic components such as stents, occluded devices, and bioprosthetic valves, which often can confound MR imaging. In these cases, CT may be of benefit.
- ▪
While higher in radiation dose, retrospective imaging allows accurate measurement of right and left ventricular size and function.
- ▪
Using technique optimization, a number of complex congenital heart lesions can be well imaged with CTA.
- ▪
Surgical corrections for congenital heart disease can be well assessed with CTA. Upper and lower extremity contrast opacification may be necessary, as well as delayed imaging, especially if intracardiac thrombi are to be excluded.
The established tests for the assessment of congenital heart disease are transthoracic (TTE) and transesophageal echocardiography (TEE), cardiac MRI, and cardiac catheterization.
Cardiac CT (CCT) is an emerging alternative because of its rapid acquisition times and post-processing robustness, but the potential radiation risks are particularly relevant for children and younger adults. Nonetheless, its use has increased prominently in the assessment of congenital heart disease.
Most intracardiac lesions can be assessed by echocardiography, and many procedures can be planned and guided with TTE, TEE, or intracardiac echocardiography, thus avoiding radiation risk and also providing Doppler assessment.
For patients with congenital heart disease, MRI is the established test for the evaluation of extracardiac surgical shunts, pulmonary vascular anomalies and complications, and aortic anomalies. This modality avoids the risk of radiation exposure and also provides flow information. Cardiac MRI also is the preferred test for quantifying right ventricular function and the degree of pulmonic insufficiency.
Clearly CCT is able to provide nonphysiologic but superb anatomic delineation of a wide range of congenital cardiac and thoracic vascular defects, and its use has increased substantially during the past decade. However, it remains unclear which lesions should be assessed by CCT, given the radiation risk-free, and widespread availability of other established modalities and the natural and proven complementarity of echocardiography and cardiac MRI.
The greatest contribution of CCT, therefore, is likely to be:
- □
In patients who cannot undergo MRI or whose MRI images are insufficient in lesions such as extracardiac shunts
- □
For the assessment of coronary anatomy, such as identification of an anomalous left anterior descending coronary artery in patients with tetralogy of Fallot
CT imaging is appropriate for a number of congenital pathologies. The most common of these are discussed in the following sections. It may be useful to categorize congenital lesions based on their level of simplicity rather than on physiology, as follows.
Simple Lesions
- □
Aorta
- •
Bicuspid aortic valve (see Chapter 18 )
- •
Coarctation (see Chapter 28 )
- •
Sinus of Valsalva aneurysm (see Chapter 28 )
- •
- □
Shunts
- •
Atrial septal defect (ASD)
- •
Ventricular septal defect (VSD)
- •
Patent ductus arteriosus (PDA)
- •
Partial anomalous pulmonary venous return (PAPVR)
- •
- □
Coronary artery
- •
Anomalous origin (see Chapter 11 )
- •
Anomalous termination (see Chapter 12 )
- •
- □
Other
- •
Cor triatriatum
- •
Ebstein anomaly
- •
Aorta
Bicuspid Aortic Valves
See Chapter 17 .
Coarctation
Coarctation of the aorta, a congenital narrowing of the aorta, accounts for approximately 5% of all congenital heart disease. The narrowing is most commonly located just distal to the origin of the left subclavian artery. Traditionally, coarctations were classified as infantile (preductal) or adult (postductal) types, but this classification is often misleading, because age of presentation has been found to be related more to the degree of narrowing and the presence of associated abnormalities than to location. The diameter of the aortic arch in adults often is normal compared with children, in whom a hypoplastic arch commonly is observed. The presence of multiple collateral arteries also is a featured adult presentation. (Collaterals arise primarily from the internal mammary and intercostal arteries, usually between the third and eighth ribs). Historically, surgical corrections ranging from end-to end reanastomosis to subclavian flap repair have been employed. Percutaneous treatment with angioplasty and stent placement are newer options. Complications of both surgical and interventional procedures include pseudoaneurysms, aneurysms, and recoarctation.
See Chapter 24, Aortic Diseases.
Sinus of Valsalva Aneurysm
See Chapter 26 , Aortic Diseases.
Shunts
Atrial Septal Defect
Atrial septal defects account for 6% to 14% of all congenital heart disease and as much as 30% to 40% of congenital heart disease in adults. There are three types of ASD :
- □
Ostium secundum ASD (70%), the most common type, is located in the region of the fossa ovalis ( Figs. 24-1 through 24-5 ).

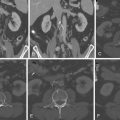
Figure 24-1
A through D, MIP contrast-enhanced four-chamber views. A secundum atrial septal defect (ASD) is present, as are right atrial and right ventricular dilation. The left heart contrast attenuation is greater than the right heart contrast due to a saline bolus passing through the right heart; the differential contrast happens to demonstrate left-to-right flow across the ASD. E and F, Three-dimensional volume-rendered views at the base of the heart. E, Absence of the mid-posterior interatrial septum, as well as dilation of the right atrium, right ventricle, and pulmonary artery. F, The interatrial septum is present at a more posterior level. There is right atrial and right ventricular dilation. Secundum ASD with right heart volume overload.

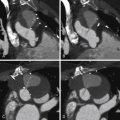
Figure 24-2
Multiple images from a cardiac CT study in a 54-year-old woman with shortness of breath on exertion and atypical chest pain. A and B, Axial source images from the CT data set. These images demonstrate an inadherent flaplike portion of the upper interatrial septum. A communication across this flap is seen ( B ) corresponding to a patent foramen ovale (PFO). C and D, Below the PFO in the midportion of the interatrial septum, a second defect which measures 1.3 cm is seen within the interatrial septum, with an appearance typical for a small to moderate-sized ostium secundum defect. The study was obtained in mid-diastole. The diagnosis was coexistent PFO and ostium secundum atrial septal defect.

Figure 24-3
Same patient as Figure 24-2 . Multiple volume-rendered images from a helically acquired cardiac CT study. A and B, Functional evaluation of the left ventricle. C and D, Functional evaluation of the right ventricle. End-diastolic images are seen in A and C and end-systolic images in B and D. In this patient with coexisting patent foramen ovale and ostium secundum atrial septal defect, stroke volumetry of the right and left ventricles generated a Qp/Qs of 1.3–1, a mild left-to-right shunt.

Figure 24-4
Multiple images from a cardiac CTA study demonstrate a small atrial septal defect (ASD). Four-chamber reconstruction ( A ) and a short-axis reconstruction across the interatrial septum ( B ). Contrast is seen shunting from left to right across the midportion of the interatrial septum. C and D, Cross-sectional localization of the ASD. On this diastolic phase prospectively acquired image, the cross-sectional measurement of the ASD is 11 × 11 mm. E and F, Volume-rendered representations of stroke-volumetry between the right ventricle ( E ) and the left ventricle ( F ). Although this study was acquired prospectively, and the latest phase within diastole was reconstructed at 80% of the R-R interval, these measurements still provide a reasonable measure of stroke volumetry. In this case a Qp/Qs by stroke volumetry was measured at 1.5 to 1.

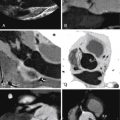
Figure 24-5
A, Four-chamber projection demonstrates a secundum atrial septal defect (ASD) with contrast extending across the ASD from left to right. B, Short-axis projection through the interatrial septum demonstrates the ASD, which measures 16 mm. C, Angioscopic projection from within the right atrium demonstrates the ASD with the pulmonary veins visualized through the ASD within the left atrium. D, Opposite angioscopic projection from within the left atrium demonstrates the ASD and the pulmonary veins. E, Volume-rendered image of the right ventricle demonstrates the right ventricular end-diastolic volume of 296 mL (enlarged). F, Volume-rendered image of the left ventricle with an end-diastolic volume of 115 mL. Assuming no valvular insufficiency, by the ratio of the end-diastolic volumes there is a flow ratio of 2.6:1. G, Curved reformation of the left anterior descending artery (LAD) demonstrates mild calcific plaque in the proximal LAD without significant stenosis. H, Curved reformation through the right coronary artery demonstrates no evidence of atherosclerosis or stenosis.
- □
Sinus venosus ASD (15%) is located above the fossa ovalis, adjacent to the superior aspect of the atrial septum, near the entrance of the superior vena cava (SVC) into the right atrium. This form of ASD is commonly associated with PAPVR of the right upper lobe pulmonary vein into the SVC ( Figs. 24-6 and 24-7 ; ).

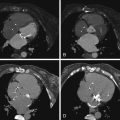
Figure 24-6
Multiple composite images from a cardiac CT study in a 52-year-old man with atypical chest pain and shortness of breath. Figure 24-7 demonstrates the presence of a sinus venosus defect, and multiple anomalous pulmonary veins The study was obtained retrospectively, in a low-dose, dose-modulated fashion. The patient’s overall dose–length product (DLP) for the study was 398 (mGy cm). A and B, Images reconstructed into four-chamber and short-axis oblique projections. A and C, End-diastole; B and D, end-systole. These images demonstrate marked dilatation of the right ventricle, and moderate right ventricular hypertrophy. E and F, A functional assessment of both the right and left ventricles. The left ventricle demonstrates normal ejection fraction at 66%. The right ventricle ( E ) also demonstrates normal systolic function with an ejection fraction of 56%. Stroke volumetry, however, demonstrates a Qp/Qs of 2.1:1. See

Figure 24-7
Same patient as Figure 24-6 . A and B, Partial anomalous pulmonary venous return (PAPVR) from the right upper lobe into the superior vena cava. C, There is more complexity to this setting of PAPVR, with a right middle lobe branch as well as a more posteriorly oriented branch from the right lower lobe—likely the superior segment—also draining into the cavoatrial junction. D, Streaming of contrast across a high superior sinus stenosis defect. This contrast extends down from the superior vena cava, and across the atrial septal defect into the left atrium. While the association of PAPVR and a superior sinus venosus defect is well documented, it is important for the cardiac CT imager to look for the presence of more than one single anomalous vein, because multiple anomalous veins may be present. See
- □
Ostium primum ASD (15%) is part of the spectrum of atrioventricular (AV) septal defects, which often are associated with incompetence of the AV valve.
Unroofed Coronary Sinus
The coronary sinus is a normal structure that is located posteriorly within the left AV groove. The confluence of the coronary sinus and the right atrium is located along the posterior inferior wall of the right atrium. In the normal state, no communication between the coronary sinus and the left atrium exists. An unroofed coronary sinus is a rare type of ASD in which the superior margin of the coronary sinus is deficient, allowing communication between the left atrium and coronary sinus to occur. This defect is commonly associated with a persistent left SVC, which is seen in approximately 0.1% to 0.5% of the general population. A small number of left SVCs (8%) drain directly into the left atrium. Associated unroofed coronary sinuses common, occurring over 70% of these patients. The morphology of the coronary sinus defect can vary, ranging from total deficiency of the roof of the coronary sinus, to a well-defined single defect between the coronary sinus and the left atrium, as well as multiple small cribriform-like fenestrations between the coronary sinus and left atrium. Coronary sinus defects also can occur in the setting of other congenital heart diseases, including tetralogy of Fallot, PAPVR, cor triatriatum, and pulmonary atresia.
The hemodynamic changes associated with ASDs depend on the size of the defect and the amount of shunting across the defect. Small ASDs with a small left-to-right shunt are well tolerated and produce few adverse hemodynamic consequences. When the ASD is large, right ventricular volume overload may occur, resulting in right atrial and right ventricular enlargement. After the age of 30, the incidence of pulmonary vascular disease increases in patients with ASDs. As pulmonary hypertension develops, a reversal of the shunt can occur, with blood flowing from the right to left across the ASD, a phenomenon known as Eisenmenger physiology.
In general, there are two ways to repair ASDs: a surgical approach with patching of the ASD and diversion of an anomalous vein if one is present. More recent advances of percutaneous occluder devices across the interatrial septum can also be used.
Although CCT is not primarily used as a diagnostic tool for ASDs, patients occasionally are unable to undergo TEE or MRI, and in this setting cardiac CT offers a valuable alternative. ASDs can be categorized and sized using CT. In addition, concomitant assessment of pulmonary vein anatomy and volumetric QP/QS quantification using right and left ventricular volumes can be derived from a single cardiac CT data set. Suitability for occluder device placement can be determined by identifying a rim of atrial tissue surrounding all margins of the ASD. Evaluation of the occluder device post-placement is better performed on CT than MRI, although small peri-device leaks are most likely best identified on TEE ( Figs. 24-8 and 24-9 ; .


Patent Foramen Ovale/Aneurysm of the Interatrial Septum
A patent foramen ovale is a result of the failure of anatomic fusion of the septum primum over the limbus of the fossa ovalis, which normally occurs when left atrial pressures exceed right atrial pressures after birth. The lack of fusion of this so-called “flap valve” of the fossa ovalis can allow left-to-right shunting. (Right-to-left shunting occurs only when right atrial pressure is higher than that on the left.) A patent foramen ovale is a common finding, present in 25% of adults.
Atrial septal aneurysms are a localized region of thinning of the interatrial septum that bulges into the right or left atrium. These are defined by bowing of the septum from the base of the interatrial septum by more than 10 or 15 mm. Most of these aneurysms are believed to be congenital in origin. Some may be caused by postsurgical remodeling of the interatrial septum from prior atrial septal surgery. The prevalence of atrial septal aneurysms is about 1% based on autopsy series. Although they are generally felt to be a benign clinical entity, atrial septal aneurysms often are fenestrated and can be associated with interatrial shunting. Cardiogenic embolism also may occur as a result of thrombus formation within the aneurysm or as a paradoxical embolism. Other congenital lesions such as patent foramen ovale and atrial septal defects also may be associated with atrial septal aneurysms. Ventricular septal aneurysms are often discovered unexpectedly as part of a cardiac CT study and are generally not the primary reason for cardiac CT study. In a patient with cryptogenic stroke, or in whom echocardiography is suboptimal, cardiac CT may be used to further evaluate for an intracardiac source of embolization. Interatrial septal aneurysms and associated shunting can be identified on cardiac CT ( Figs. 24-10 through 24-15 ; ).