Key points
- •
Skull base fractures are managed based on associated intracranial injury and complications, including vascular and cranial nerve injury and cerebrospinal fluid (CSF) leak.
- •
Anterior cranial fossa fractures, particularly comminuted and oblique frontobasal fractures, are commonly associated with CSF leak, either acute or delayed in presentation.
- •
Transverse middle cranial fossa fractures extending through the carotid canal are at increased risk for vascular injury, and should prompt screening with vascular studies, such as CT angiography.
- •
Thin-section multiplanar CT reformations, as well as 3-dimensional reconstructions, are helpful in the detection of subtle skull base fractures.
Introduction
Head trauma is one of the most common reasons for visits to the emergency department in the United States. According to the 2013 National Trauma Data Bank maintained by the American College of Surgeons, of 833,311 adult trauma admissions reported from 805 facilities across the United States, approximately 36% sustained an injury to the head. Skull base fractures, those fractures that extend through the floor of the anterior, middle, or posterior cranial fossa, occur in an estimated 7% to 16% of nonpenetrating head injuries, and are due to a relatively high-velocity trauma, most often high-speed motor vehicle accidents, although motorcycle collisions, pedestrian injuries, falls, and assault are additional associated causes. Penetrating trauma, particularly gunshot wounds, are seen much less frequently, accounting for less than 10% of cases.
Skull base injury is often seen in the setting of complex facial or orbital fractures, and detection of basilar skull fractures is important, as even linear nondisplaced fractures can be associated with numerous critical complications, including intracranial and orbital injuries, cerebrospinal fluid (CSF) leak, cranial nerve palsies, and vascular injuries. Although facial fractures often require repair to improve function and cosmesis, the management of patients with skull base injury is dependent on the extent of associated intracranial injury and other complications. The associated risk and extent of complications often depends on the location and pattern of the fracture, which is in turn determined by the mechanism of injury and type of impact.
Introduction
Head trauma is one of the most common reasons for visits to the emergency department in the United States. According to the 2013 National Trauma Data Bank maintained by the American College of Surgeons, of 833,311 adult trauma admissions reported from 805 facilities across the United States, approximately 36% sustained an injury to the head. Skull base fractures, those fractures that extend through the floor of the anterior, middle, or posterior cranial fossa, occur in an estimated 7% to 16% of nonpenetrating head injuries, and are due to a relatively high-velocity trauma, most often high-speed motor vehicle accidents, although motorcycle collisions, pedestrian injuries, falls, and assault are additional associated causes. Penetrating trauma, particularly gunshot wounds, are seen much less frequently, accounting for less than 10% of cases.
Skull base injury is often seen in the setting of complex facial or orbital fractures, and detection of basilar skull fractures is important, as even linear nondisplaced fractures can be associated with numerous critical complications, including intracranial and orbital injuries, cerebrospinal fluid (CSF) leak, cranial nerve palsies, and vascular injuries. Although facial fractures often require repair to improve function and cosmesis, the management of patients with skull base injury is dependent on the extent of associated intracranial injury and other complications. The associated risk and extent of complications often depends on the location and pattern of the fracture, which is in turn determined by the mechanism of injury and type of impact.
Normal anatomy
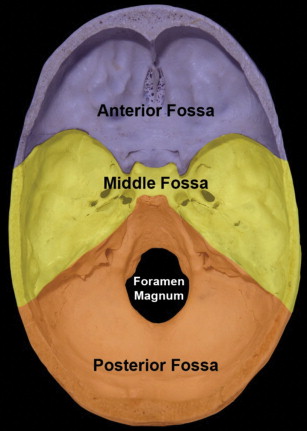
The skull base is made up of 7 bones, the paired frontal and temporal bones, and the unpaired ethmoid, sphenoid, and occipital bones. It is divided into anterior, central, and posterior regions, which form the floor of the anterior, middle, and posterior cranial fossae.
The anterior skull base, formed by the frontal and ethmoid bones, separates the anterior and inferior frontal lobes and olfactory structures within the anterior cranial fossa from the orbits and the sinonasal cavity. The lateral and anterior borders of the anterior cranial fossa are formed by the orbital plate of the frontal bone and the posterior table of the frontal sinus. Inferiorly, the floor of the anterior cranial fossa is formed by the cribriform plates and roof of the ethmoid sinuses. The posterior border between the anterior and central skull base is formed by the lesser wing of the sphenoid bone, including the clinoid process, and the planum sphenoidale ( Fig. 1 ).
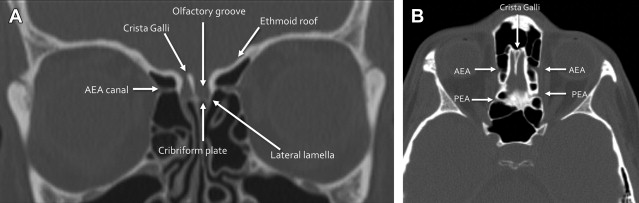
Deep clefts lateral to the midline crista galli form the olfactory grooves, which house the olfactory bulbs. The floor of the olfactory groove is formed by the cribriform plates, which are inherently thin, with multiple small foramina through which the small branches of the olfactory nerve pass. The lateral lamella is a thin bone connecting the cribriform plate with the fovea ethmoidalis, or the roof of the ethmoid sinuses, all part of the ethmoid bone. In addition to the cribriform plate foramina, the anterior skull base contains the anterior and posterior ethmoid artery foramina, which should not be confused with fractures; these may represent significant sources of epistaxis, if injured ( Fig. 2 ).
The central skull base, formed by the sphenoid and anterior temporal bones, separates the pituitary gland (within the sella), the cavernous sinuses (including the carotid artery and cranial nerves), the Meckel cave, and the temporal lobes superiorly from the sphenoid sinus anteriorly and inferiorly, and the extracranial soft tissues deep to the skull base inferiorly, including the masticator, parotid, parapharyngeal, and pharyngeal mucosal spaces. The anterior border of the central skull base is formed by the posterior margin of the lesser wing of the sphenoid bone, clinoid process and tuberculum sella. The floor is formed by the greater wing and central body of the sphenoid bone, the sphenoid sinus, and the sella. The posterior border between the central and posterior skull base is formed by the superior margin of the petrous ridge of the temporal bone, the basi sphenoid portion of the clivus, and the dorsum sella (see Fig. 1 ). In addition to housing the pituitary gland, the central skull base contains numerous foramina and canals through which many important structures pass, including cranial nerves (CNs) II to VI and the internal carotid artery ( Fig. 3 , Table 1 ).
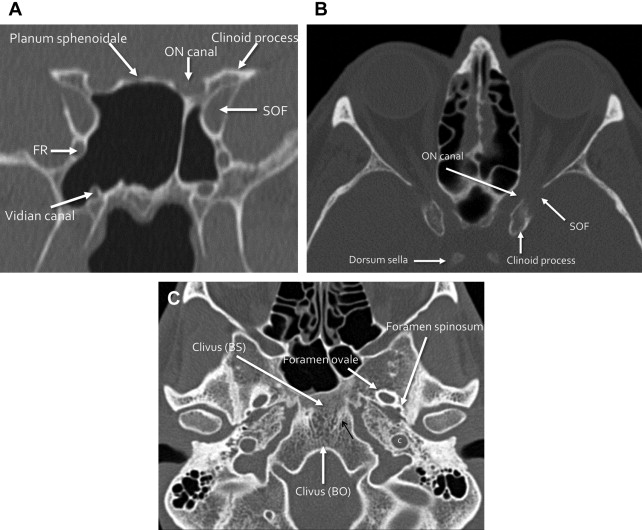
| Foramen | Contents |
|---|---|
| Optic nerve canal | CN II (optic nerve) Ophthalmic artery |
| Superior orbital fissure | CN III (oculomotor nerve) CN IV (trochlear nerve) CN V1 (ophthalmic branch of the trigeminal nerve) CN VI (abducens nerve) Superior ophthalmic vein |
| Foramen rotundum | CN V2 (maxillary branch of trigeminal nerve) |
| Foramen ovale | CN V3 (mandibular branch of trigeminal nerve) |
| Carotid canal | Internal carotid artery Sympathetic plexus |
| Foramen spinosum | Middle meningeal artery |
| Vidian canal | Vidian nerve and artery |
| Foramen lacerum | Cartilage |
The posterior skull base is formed by the posterior temporal bone and the occipital bone, and separates the posterior fossa structures, including the cerebellum and brainstem, from the extracranial soft tissues: the posterior nasopharynx, retropharyngeal space, carotid space, and perivertebral space. The anterior border is formed by the petrous ridge of the temporal bone superiorly, and the clivus (basi occiput portion) inferiorly. The inferior border includes the occipital condyles and the mastoid portion of the temporal bone, and the posterior skull base extends posteriorly to the squamous portion of the occipital bone (see Fig. 1 ). Some consider the temporal bone proper to be the lateral, or posterolateral skull base.
The largest foramen of the skull base, foramen magnum, is located within the posterior skull base, and transmits the medulla oblongata (cervicomedullary junction), vertebral arteries, and spinal portion of CN XI. Other major foramina within the posterior skull base include the internal auditory canal (CN VII, VIII, and labyrinthine artery), jugular foramen (pars nervosa anteriorly: CN IX, inferior petrosal sinus, and Jacobsen nerve; pars vascularis posteriorly: CN X, XI, Arnolds nerve and jugular bulb), and hypoglossal canal (CN XII) ( Figs. 4 and 5 ).
Pathology
Fractures through the skull base are often the sequelae of high-velocity impact and may be linear or comminuted, with a potentially complex imaging appearance. Fracture patterns and associated complications depend on the location of the injury, which is often determined by the mechanism of injury and type of impact. Table 2 outlines the most commonly encountered complications associated with site of injury.
| Location | Complication |
|---|---|
| Anterior skull base | Intraorbital injury |
| Sinonasal CSF leak/meningoencephalocele | |
| Anosmia (CN I injury) | |
| Central skull base | Vascular injury (ICA occlusion, dissection, pseudoaneurysm, aneurysm, CCF) |
| Cranial nerve injury (optic nerve, CN III, IV, V, and VI) | |
| Horner syndrome | |
| Posterolateral skull base (temporal bone) | Vascular injury (ICA) |
| CN VII or VIII injury | |
| Mastoid CSF leak/meningoencephalocele | |
| Posterior skull base | Venous vascular injury or vertebrobasilar injury |
| Lower cranial nerve injuries (CN IX, X, XII, or XII) | |
| Craniocervical junction and cervical spine injuries |
Anterior Skull Base Fractures
Classification/Etiology
Direct frontal trauma often results in anterior skull base fractures, so-called frontobasal injuries, with the “frontal” component of fractures involving the upper facial third (frontal bone/sinus and superior orbital rim), and “basal” component of fracture involving the anterior skull base (cribriform plate, ethmoid roofs, and planum sphenoidale). Numerous classification systems for frontobasal injuries have been described in an attempt to stratify complications and guide management. One of the more recent anatomic classification systems evolved based on cadaveric studies demonstrating unique reproducible fracture patterns, similar to Lefort facial fractures, and classifies frontobasal fractures into types I through III.
Type I frontobasal fractures are generally associated with a relatively lower impact frontal injury, and are defined as linear fractures that initially parallel the cribriform plate, then may extend posteriorly along the sella and petrous ridge to separate the anterior and middle cranial fossa from the posterior cranial fossa. These generally are more medially located, involving the medial third of the supraorbital rim ( Fig. 6 ), and are less frequently associated with complications. Type II fractures are composed of more lateral vertical linear fractures of the frontal calvarium and anterior skull base and often extend to involve the lateral two-thirds of the supraorbital rim, the squamous portion of the temporal bone, the orbital roof, the lateral orbital wall, or the orbital apex. These are more frequently associated with CSF leak and intracranial injury ( Fig. 7 ). Finally, type III fractures combine central and lateral frontobasilar fractures, often with comminution of the entire frontal bone, orbital roof, and lateral cranial vault ( Fig. 8 ). Type II and III fractures are more frequently associated with concomitant midface injuries and are thought to be related to a higher-velocity impact from a lateral or inferior frontal or supraorbital vector. As one might suspect, because type III fractures are associated with the greatest force, they are most often associated with complications such as intracranial injury and CSF leak, reportedly in up to 25% of cases.
One additional classification system, devised by Piccirilli and colleagues, divides frontobasal fractures into types A to C, each denoting a different surgical approach for management. Type A fractures involve only the anterior table of the frontal sinus, whereas type B fractures involve the posterior table ( Fig. 9 ). Type C fractures include any frontobasal injury that does not involve the frontal sinus. Fractures extending through the posterior table of the frontal sinus (type B), particularly when comminuted, in addition to potentially causing CSF leak, can ultimately result in the development of a mucocele, due to entrapped mucosa along the fracture line. Thus, these fractures may require a different surgical approach, including frontal sinus obliteration (removing the mucosa and filling the sinus with fat or other materials, such as hydroxyapatite) or cranialization (removal of the posterior table mucosa and bone, and performing any duraplasty needed at the time). Both of these surgeries should involve surgical obstruction of the outflow tracts. Some investigators currently advocate cranialization if the fracture involves more than 25% of the posterior table of the frontal sinus. Less displaced fractures can be managed conservatively, or via endoscopic approaches.
Complications
The management of skull base fractures is based on anticipated potential complications. Although many facial fractures may require repair to maintain function and cosmesis, skull base fractures often require repair only if there is associated intracranial injury requiring decompression, persistent CSF leak, or significant cranial nerve or vascular injury. Also, extensive fractures through the posterior table of the frontal sinus may require surgical repair to prevent mucocele formation. Anterior skull base injuries are frequently seen in conjunction with frontal lobe contusions and intraorbital injuries, which are discussed in the article by Gupta and colleagues, elsewhere in this issue. The most commonly associated complications are CSF leak (with or without meningitis), and injury to the olfactory nerve, resulting in anosmia; these are unique to the anterior skull base.
CSF leak after trauma occurs when there is both an osseous defect and a tear of the closely adherent dura, leading to egress of CSF from the subarachnoid space into the sinonasal cavity (in the setting of frontobasal fractures) or into the middle ear cavity and mastoid air cells (in the setting of temporal bone trauma). CSF rhinorrhea or otorrhea is the usual clinical presentation. There are rare reports, in the setting of complex orbital roof injuries, of oculorrhea and intermittent periorbital swelling and tearing due to accumulation of CSF. The more comminuted and displaced fractures, such as type III frontobasal fractures, carry the greatest risk of CSF leak. Communication with the flora of the nasal or middle ear cavities results in meningitis, reportedly in up to 50% of cases, if the leak is not repaired. The risk is approximately 1% in the first 24 hours, increasing to 18% at the end of 2 weeks ( Fig. 10 ). In fact, any patient presenting clinically with recurrent episodes of meningitis (particularly in the setting of prior trauma) should be evaluated for occult CSF fistula.
Traumatic CSF leak, which is the most common form of CSF leak, occurs in 10% to 30% of skull base fractures, and most often presents as CSF rhinorrhea (in 80% of cases). Eighty percent of cases will present in the first 48 hours after injury, and most (up to 95%) will present with CSF rhinorrhea in the first 3 months after trauma. The delay in symptom onset is primarily due to resolution of the initial posttraumatic edema and hemorrhage, combined with increased mobility as the patient progresses through rehabilitation. However, a small minority of patients (5%) will present in a more delayed fashion, months to even decades after their trauma, possibly due to fracture fragments slowly eroding and thinning the dura over time ( Fig. 11 ).
Most posttraumatic CSF leaks, up to 85%, are acute in presentation, and heal spontaneously with conservative management such as bed rest, head elevation, and stool softeners. Occasionally patients will require CSF diversion with lumbar drain or external ventricular drain placement if the leaks do not resolve in approximately 2 to 7 days. One recent study demonstrated good results in high-risk patients with the administration of acetazolamide in the early posttraumatic period. Of course patients presenting with acute CSF rhinorrhea with intracranial pathology requiring immediate surgical treatment, such as frontal lobe hematomas or depressed skull fractures, will undergo open intracranial repair of fractures and duraplasty at the time of the surgery. Those with persistent leaks for longer than 7 to 10 days, in spite of CSF diversion, often require repair, either intracranial or endoscopic. Larger skull base defects (>1.5 cm) or severely comminuted fractures, particularly if a meningoencephalocele is present, are associated with a worse prognosis for spontaneous resolution, and will require surgical repair. The use of prophylactic antibiotics to avoid meningitis is a controversial topic. A recent large meta-analysis reviewing the outcomes of 1241 patients showed overall no statistically significant decrease in meningitis in patients treated with prophylactic antibiotics. Similarly, a Cochrane review of 2168 patients in 2011 showed no evidence to support the use of prophylactic antibiotics, but recommended the need for large randomized controlled trials in the future.
Loss of smell, or anosmia, as a result of olfactory nerve injury, is a relatively common complication after trauma, with an overall incidence of up to 7%, and an increased risk in anterior skull base fractures, particularly medial fractures along the cribriform plates. Traumatic CSF leak, particularly when repair is required, has been associated with an increased risk of anosmia as well. Only approximately 10% of all patients with traumatic anosmia are estimated to recover sense of smell, often in a delayed fashion, months to years after the injury.
Central Skull Base Fractures
Classification/Etiology
Extension of frontobasal fractures from frontal impact can occur in a sagittal or oblique fashion through the central skull base with involvement of the sella and sphenoid sinus, and, in some cases, the temporal bone ( Fig. 12 ). Transverse fractures in a coronal plane through the central skull base are common and are usually the result of high velocity lateral impact to the lateral frontal bone, zygoma, temporal, or parietal bone. Several transverse fracture patterns have been described, including transverse or oblique/diagonal patterns; these are related to the mechanism of injury. Transverse fractures extend through the sphenoid sinus, either anteriorly or posteriorly, depending on type of impact, and can propagate laterally through the greater sphenoid wing and squamosal temporal bones, or be associated with temporal bone fractures ( Fig. 13 ). The more anterior transverse fractures often involve the posterior aspect of the anterior cranial fossa (posterior ethmoid roof) and may extend through the clinoid processes, involving both the orbital apex and the superior orbital fissures and potentially resulting in cranial nerve or vascular injuries. The more posterior transverse patterns may involve the temporal bones bilaterally, extending along the sphenopetrosal synchondrosis, with involvement of the posterior sphenoid sinus and/or clivus ( Fig. 14 ). Oblique central skull base fractures, often associated with facial fractures and type II or III frontobasal fractures, are more often associated with CSF leak. Transverse fractures are more commonly associated with vascular and cranial nerve injuries, although these can be seen in both types of central skull base fractures, depending on their course.
Involvement of the clivus with central and posterior skull base fractures deserves special mention. These fractures are rare, accounting for about 2% of all cranial fractures, but are associated with high mortality (24%–80%) because of location and proximity to the brainstem, as well as high incidence of neurologic and vascular injury (up to 46%). There are 2 characteristic patterns of clival injury: transverse or oblique, and longitudinal. Transverse or oblique fractures, similar to other transverse central skull base fractures, are caused by a lateral or crushing injury, and are commonly associated with cranial nerve and internal carotid artery (ICA) injury ( Fig. 15 ). Conversely, longitudinal clival fractures are more complex, are due to axial loading mechanism from the vertex, and are often associated with vertebrobasilar injury and brainstem infarction. Longitudinal clival fractures are seen in conjunction with craniocervical junction injuries, and retroclival hematomas. A hallmark of clival fracture clinically is the presence of sixth nerve palsy, due to the location of the Dorello canal within the clivus. Other cranial nerve injuries are also frequently seen.
Complications
Central skull base fractures are primarily managed based on associated complications. Many are associated with intracranial injuries, including multicompartmental hemorrhage, temporal lobe parenchymal contusions, and diffuse axonal injury (DAI), as these injuries are associated with high-velocity impact ( Fig. 16 ). Anterior middle cranial fossa epidural hematomas, also called benign venous epidural hematomas, occur in conjunction with injury to the greater wing of the sphenoid bone. These are postulated to be a result of venous injury to the sphenoparietal sinus, and have a benign self-limited course, not requiring surgical drainage ( Fig. 17 ). CSF leaks caused by fracture extension through the sphenoid sinus occur less frequently than in the frontobasal region, but are common with comminuted fractures.