Usual course: 8–10 months of rapid growth shortly after birth followed by involution
US
High vascular density (5 vessels/cm2)
Many surrounding vessels have low-resistance waveforms
Usually hypoechoic and well defined
MRI
Intense, diffuse enhancement until involution
No calcifications or phleboliths
Hemangiomas and vascular malformations were differentiated in 1982 [12]. Infantile hemangiomas are encountered in over 12 % of infants, most often in girls and more commonly in fair-haired children [10]. The infantile hemangioma is small or absent at birth and then enters a proliferative phase over the first 8–10 months, during which it grows rapidly (under the influence of vascular endothelial growth factor and fibroblast growth factor). It then enters a plateau phase that lasts for months to several years, followed by an involution phase, when it gradually shrinks. It usually involutes completely within 10–12 years, and 50 % are completely gone within the first 5 years of life [13]. Children with multiple cutaneous hemangiomas are more likely to have multiple visceral hemangiomas [13]. Lesions on the skin surface are usually clinically obvious due to their “strawberry” appearance, but deeper lesions may manifest only by bluish discoloration of the skin (due to overlying draining veins) or mass effect.
The most appropriate treatment for these lesions is usually watchful waiting and reassurance, unless the lesion interferes with the child’s function, such as blocking the airway or eyes, and in cases of rapid growth, ulceration with hemorrhage, visceral involvement, or congestive heart failure. Up to 10 % of patients with infantile hemangiomas require treatment [14]. Initial treatment is usually with high-dose systemic corticosteroids; 30 % of hemangiomas respond dramatically, and 40 % have some response [15]. Some centers have begun using propranolol as a first-line agent to treat infantile hemangiomas, as it is safer and, in some studies, more effective [16]. Other treatment modalities include antiangiogenesis factors such as α-interferon and chemotherapeutic agents such as vincristine, though the side effects of these treatments are substantial. Embolization is used for life-threatening complications, such as bleeding, congestive heart failure, or airway compression [11].
Of note, Kasabach-Merritt syndrome does not occur in the typical infantile hemangioma. This syndrome, which consists of thrombocytopenia, anemia, and consumptive coagulopathy, is associated with the rare variant called tuft hemangioma (which resolves more slowly than the common infantile hemangioma) and with kaposiform hemangioendothelioma (see section below) [11].
Imaging
The initial imaging modality for a superficial infantile hemangioma is usually visual inspection, followed by expectant management. If the lesion is deep, or if the diagnosis is uncertain, US is the most appropriate first imaging study due to cost, speed, and availability. A hemangioma usually appears well defined, solid, and hypoechoic relative to subcutaneous fat [17] (Fig. 22.1). It occasionally appears hyperechoic or heterogeneous [18]. High vessel density, defined as more than five vessels per cm2 in the part of the lesion with highest flow on Doppler imaging, is typical [17]. The lesions usually have multiple surrounding vessels demonstrating low-resistance Doppler flow [15]. A maximum Doppler shift of greater than 2 kHz has been proposed as a criterion for diagnosis of hemangioma, but the utility of this finding is unclear [17]. US can usually confirm the diagnosis of hemangioma, though it may not demonstrate the full extent of the lesion.
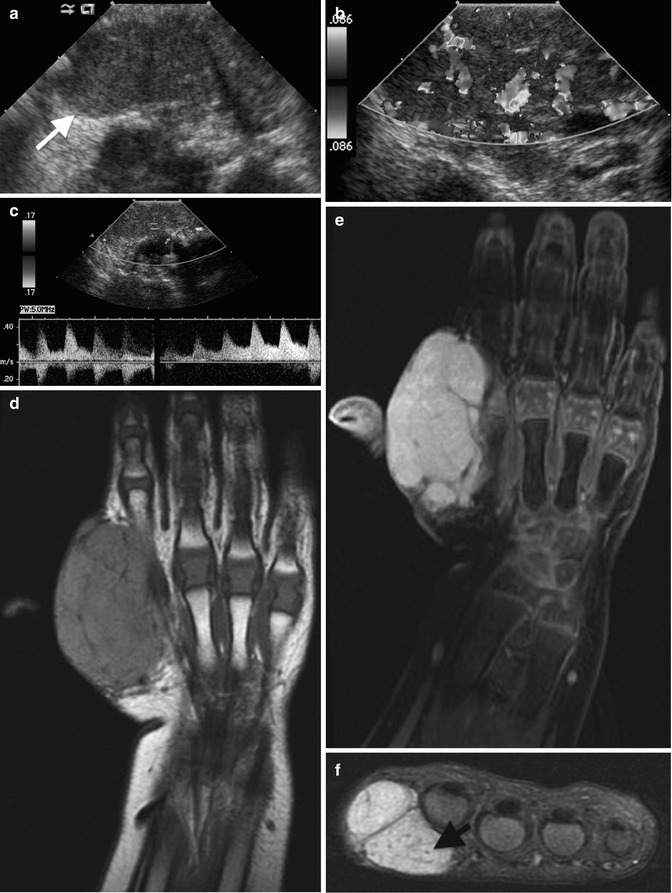
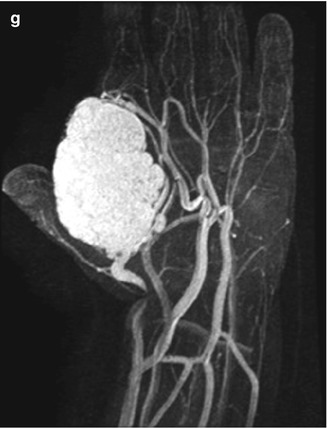


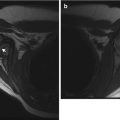
Fig. 22.1
Hemangioma of the hand in a 13-month-old boy. (a) Ultrasound (US) shows a hypoechoic, homogenous, and lobulated superficial soft tissue mass (arrow). (b) Doppler US shows high vessel density. (c) Spectral Doppler interrogation shows low-resistance arterial waveforms. The mass is intermediate signal on coronal T1-weighted (T1-W) magnetic resonance imaging (MRI) (d), and it enhances intensely (e, coronal T1-W FS gadolinium-enhanced image). (f) Proton density (PD) fat suppressed (FS) axial image shows central dots representing fibrofatty septa or thrombosed vessels (arrow). (g) Coronal magnetic resonance (MR) angiography shows adjacent large high-flow vessels
Extensive lesions or lesions that cannot be fully evaluated with US should be evaluated with MRI. The MRI appearance varies with the phase of the lesion. During proliferation and plateau phases, hemangiomas appear isointense to muscle on T1- and hyperintense on T2-W imaging. On T2-W imaging, the typical appearance is a focal, well-marginated, lobulated mass, but margins may be indistinct at T1-W imaging. Enhancement is homogeneous and diffuse. Large, high-flow vessels, representing both arteries and veins within and around the mass, demonstrate increased signal on gradient recall echo and signal voids on spin-echo sequences; these vessels are easily seen on MR angiography. Central low-signal-intensity dots are common, probably representing fibrofatty septa viewed in cross section or perhaps thrombosed vascular channels [19]. There should be no calcifications or phleboliths. The mass may cause smooth saucerization of adjacent bone. As the lesion involutes, the vascularity and enhancement decrease, and the lesion is replaced by fat until only a few scattered hyperintense vessels remain. It is important to evaluate neurovascular, visceral, muscular, and osseous involvement, especially if embolization is contemplated [11].
Differential Diagnosis
Differential considerations include neonatal soft tissue sarcomas, such as rhabdomyosarcoma and infantile fibrosarcoma. However, sarcomas tend to demonstrate more poorly defined margins and a more heterogeneous appearance both before and after contrast administration. The triad of lobulation, septation, and central low-signal-intensity dots is more common in hemangiomas [19]. Both T2 signal and enhancement are also generally more intense in hemangiomas than in soft tissue sarcomas [19]. Indeterminate masses require biopsy, but bleeding from a biopsied hemangioma can be profuse [20]. Unusual hemangiomas include rapidly involuting, non-involuting, and intramuscular subtypes.
Vascular Malformations
Vascular malformations, unlike classic infantile hemangiomas, are composed of dysplastic vessels, are present at birth, grow proportionally with the child, and do not spontaneously regress. The lesions are classified pathologically by what types of vessels are present, but a more practical differentiation is whether the lesion has high or low flow [11], as this determines treatment.
High-Flow Lesions
Arteriovenous Malformation
High-flow arteriovenous malformations (AVMs) occur when abnormal connections develop between arteries and veins, bypassing a capillary bed [21]. They are lined with nonproliferative endothelium [22]. The central feature of an AVM is the nidus (Latin for “knot”) – a tangle of tortuous, dysplastic vessels, usually near the center of the AVM, where shunting of blood from the arterial to the venous system occurs [23].
The lesion presents as a soft, usually painless mass, often present at birth. Rarely, AVMs cause pain with exercise, warmth in the overlying skin, or distal ischemia due to steal phenomenon; compressive neuropathy, skin ulceration, high-output heart failure, or bleeding are even more unusual [22, 23]. Although most lesions grow proportionally with the child, some exhibit sudden, rapid growth in response to trauma, puberty, infection, or pregnancy [22].
The most effective treatment for an AVM is trans-arterial embolization, though surgery is occasionally necessary if embolization cannot be performed [22, 24]. An AVM may recur even after apparently successful embolization, ultimately requiring surgical resection or amputation [24].
Imaging
Radiographs may demonstrate overgrowth of adjacent bone and soft tissue due to increased blood flow (Fig. 22.2). US findings include multiple dilated vascular structures, some with high-velocity venous flow and others with low-resistance arterial flow. The nidus of the AVM may be difficult to identify [17]. The main differentiating factor between a hemangioma or venous malformation and an AVM on ultrasound is the lack of a discrete mass in an AVM.
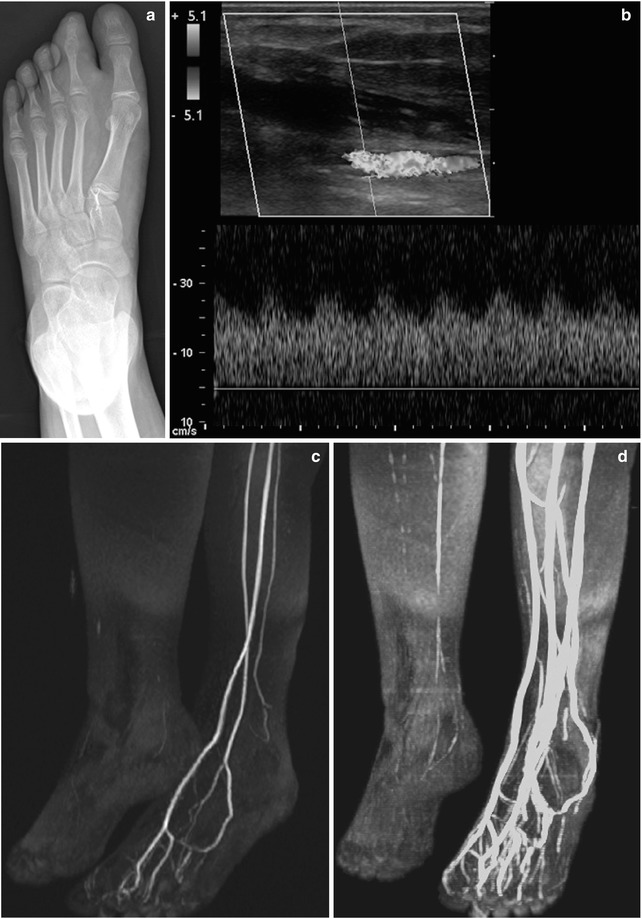
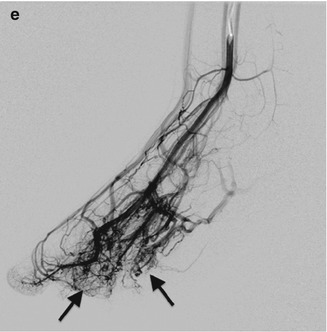


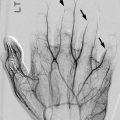
Fig. 22.2
Arteriovenous malformation (AVM) in a 10-year-old girl with a large second toe. (a) Soft tissue hypertrophy at medial foot (note hyperdense glue from attempted embolization). (b) Spectral Doppler US of the medial plantar artery shows low-resistance arterial waveform. (c) Maximum intensity projection (MIP) of gadolinium bolus MR angiography shows large feeding vessels, although the nidus is not identified. (d) Slightly delayed MIP image of gadolinium bolus MR angiography shows early draining veins. (e) Catheter digital subtraction angiography (DSA) shows complex, spiderweb-like nidus (arrows) with multiple early draining veins
MRI demonstrates multiple flow voids on spin-echo imaging and multiple surrounding venous structures, but there should be no discrete mass [22]. MR angiography can demonstrate high-flow feeding vessels. There may be overlying skin thickening or fatty overgrowth of the surrounding tissues, and there is occasionally surrounding soft tissue edema and enhancement that may simulate a mass [23]. When the lesion is confined to a muscle sheath, it closely mimics the appearance of a vascular tumor [23]. Angiography demonstrates arteriovenous shunting and demonstrates the nidus of the lesion to best effect. However, this modality is usually reserved for intervention and pre-intervention planning.
Arteriovenous Fistula
An arteriovenous fistula (AVF) consists of a direct connection between an artery and a vein. In the child it is usually iatrogenic or caused by fracture or blunt trauma [25, 26]. Clinical exam reveals a mass with a palpable thrill. US interrogation shows dilated venous structures with high-velocity flow and low-resistance arterial waveform on Doppler imaging [26]. Angiography with arterial injection reveals sudden filling of dilated veins at the level of the fistula without an intervening capillary phase. There may be associated pseudoaneurysms or other evidence of vascular trauma [26].
Low-Flow Lesions
Venous Malformation (Box 22.2)
Box 22.2: Venous Malformation
Most common soft tissue tumor in infants and children | |
Phleboliths pathognomonic | |
Most lesions contain both venous and lymphatic tissue | |
Speed of flow is more important than histology | |
Doppler US | Low-resistance arterial and venous flow |
MRI | Dilated tubular structures may enhance |
Phleboliths hypointense on all sequences | |
Venous malformations (synonymous with cavernous hemangioma and phlebangioma) are the most common soft tissue tumor in infants and children [27]. These collections of dilated venous structures that lack mural smooth muscle cells [23] are also the most common type of vascular tumor overall.
This lesion may present as a soft, easily compressible bluish mass that enlarges with the Valsalva maneuver or if positioned dependently. It may involve only the skin or may extend into underlying fat, muscle, and bone [23]. The venous malformation often causes pain that increases from infancy into adulthood [11], along with deformity and decreased range of motion. A limb with a venous malformation is more likely to demonstrate undergrowth than overgrowth [23], but there may be associated fatty overgrowth. When the mass extends into a joint, recurrent hemorrhage may result in hemosiderin arthropathy, similar to that associated with hemophilia. The mass consists of multiple thin-walled venous channels without smooth muscle cells in the walls and possibly areas of organizing thrombus [10].
Treatment may include elastic compression garments, percutaneous injection with sclerosing agents, or surgical resection [11]. Percutaneous sclerosis often requires a staged procedure with multiple sequential treatments. Only 20 % of patients will have complete relief from a single treatment, though in an additional 60 % symptoms and quality of life do improve [11].
Imaging
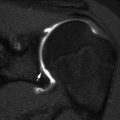
Phleboliths are the most specific radiographic finding of a venous malformation and allow definite differentiation from the classic infantile hemangioma [23] (Fig. 22.3). However, they occur in only 9–16 % of venous malformations [17]. If the lesions involve adjacent bone, the bone demonstrates lacy lytic changes that weaken trabeculae, predisposing to pathologic fractures [23].
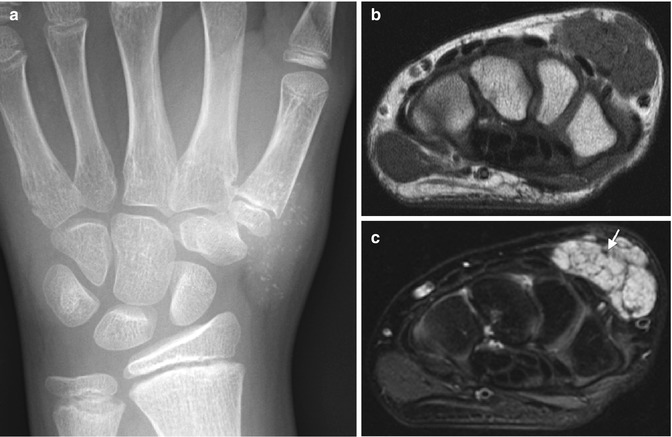

Fig. 22.3
Venous malformation in a 12-year-old boy with a wrist mass. (a) There are multiple round calcifications and ill-defined increased soft tissue density at the radial aspect of the wrist. (b) Axial T1-W image shows a lobulated, homogeneous mass of intermediate signal intensity. (c) Axial T2-weighted (T2-W) FS image shows homogeneous high signal intensity with septations and a few focal, round hypointensities (arrow) that correspond to calcifications
US demonstrates a complex mass, often with multiple slow-flowing channels with intervening septations. There may be areas of low-resistance arterial flow, with antegrade flow both at systole and diastole (Figs. 22.4 and 22.5). Large phleboliths demonstrate acoustic shadowing [27]. Computed tomography (CT) reveals a soft tissue mass with multiple enhancing serpentine vascular structures [27]. There may be associated fatty overgrowth.
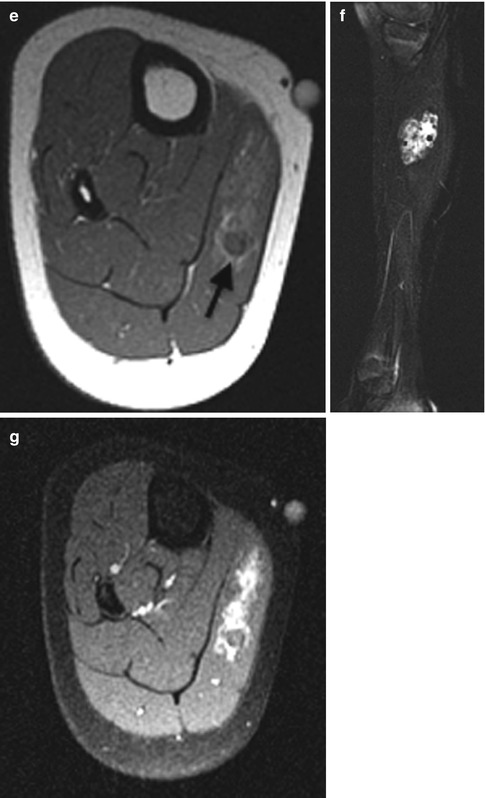
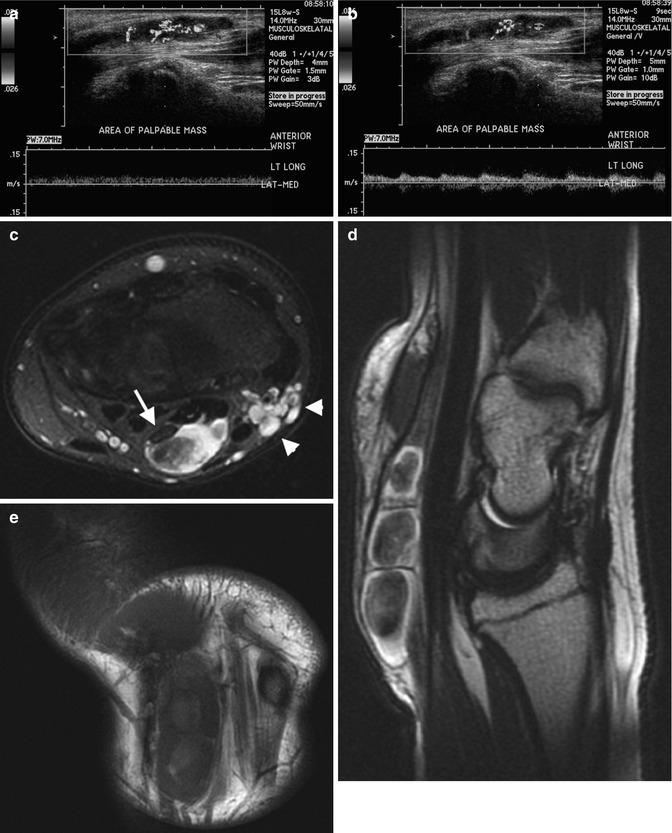


Fig. 22.4
Venous malformation in a 12-year-old girl complaining of calf pain. (a) US shows a heterogenous mass with shadowing, echogenic phleboliths. (b) Color Doppler US shows high vessel density. (c) Spectral Doppler waveform shows low-resistance arterial flow, compared with (d) normal triphasic arterial waveform of the anterior tibial artery. (e) Axial T1-W image shows a mass of intermediate signal intensity in the medial head of the gastrocnemius. Note phlebolith (arrow). (f) T2-W FS image shows heterogeneous hyperintensity. (g) Axial T1-W FS image shows homogeneous enhancement (except at the phlebolith)

Fig. 22.5
Venous malformation of the wrist in a 15-year-old boy. The mass had multiple dilated vessels and foci of organizing thrombus and was intimately related with the median nerve. Spectral Doppler images demonstrate low-resistance venous (a) and arterial (b) flow within the mass. (c) Axial T2-W FS image shows central low signal, which corresponded to organizing thrombus. Note proximity to median nerve (arrow), which might suggest the diagnosis of nerve sheath tumor. There are enlarged vessels at the radial aspect of the wrist (arrowheads). (d) Sagittal T2-W FS image shows striking central hypointensity. (e) Coronal T1-W image shows multiloculated hypointense mass
Small lesions appear homogeneous at MRI, with intermediate- to high-signal intensity on T1-W imaging and high signal on T2-W imaging (Fig. 22.5). Especially if the lesion is large, there is often overgrowth of surrounding fat. Fluid-fluid levels are rare and may correspond to areas of hemorrhage [23] (Fig. 22.6). Surrounding enlarged low-flow vessels may appear as dilated, fluid-filled (high T2, intermediate to low T1), tubular structures. After gadolinium administration, these vessels may enhance due to their low-velocity flow, unlike the high-flow vessels in AVMs and AVFs, which appear as flow voids [23]. Phleboliths appear as rounded areas of low signal on all sequences [21]. However, it should be noted that both venous and lymphatic components can coexist within a single malformation – in fact, more lesions demonstrate mixed histology than exist as pure venous or lymphatic malformations [11]. The lymphatic components do not enhance after contrast administration. Fortunately, the exact histology is less important than the speed of flow.
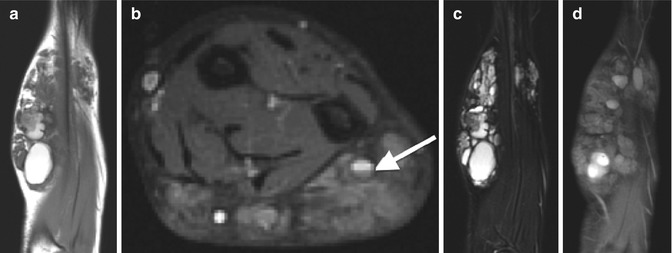

Fig. 22.6
Venous malformation involving the forearm in an 11-year-old boy. (a) Coronal T1-W image of the forearm shows a complex mass, predominantly isointense to muscle, with rounded areas of high signal and a hypointense rim corresponding to hemorrhage. Subcutaneous fat is overgrown. (b) Axial T2-W FS image shows high signal and septations with a fluid-fluid level (arrow). (c) Coronal T2-W FS image shows hyperintensity with multiple septations. (d) Coronal T1-W FS gadolinium-enhanced image shows diffuse low-level enhancement with areas of hyperintense hemorrhage and intervening lobules of overgrown subcutaneous fat
It is important to evaluate the extent to which the mass involves adjacent neurovascular bundles when planning for surgery, and if the lesion involves a joint, it is important to carefully assess the articular cartilage for evidence of arthropathy [23].
Lymphatic Malformation (Box 22.3)
Box 22.3: Lymphatic Malformation
Invasive | |
Microcystic | Nonspecific solid mass |
Macrocystic | Multiloculated cystic structure |
MRI | Complex signal denotes prior hemorrhage or infection |
Fluid-fluid levels | |
Enhancement of septations but not of cysts | |
Lymphatic malformations are composed of dilated endothelium-lined channels containing lymphatic fluid [11]. Older terms for lymphatic malformations include lymphangioma and cystic hygroma. Seventy-five percent of lymphatic malformations involve the head and neck [23]. The lesions present as a soft mass with either diffuse or localized swelling and adjacent soft tissue and skeletal overgrowth [23]. Most present in infancy or childhood, 90 % before age 2 [11]. Although usually treated with surgical excision, percutaneous sclerotherapy is occasionally employed.
Imaging
Radiographs may demonstrate lacy infiltration of adjacent bones, along with soft tissue edema and hypertrophy. At US, macrocystic lesions appear multiloculated and cystic, with thin septations (Fig. 22.7); microcystic lesions appear solid and hyperechoic. CT demonstrates a nonspecific soft tissue mass.
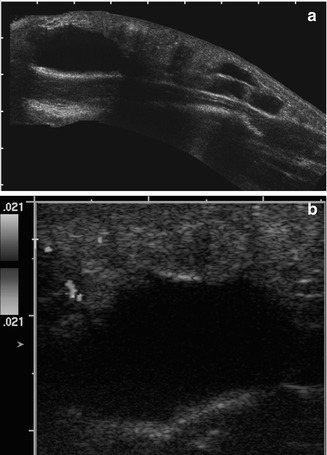

Fig. 22.7
Lymphatic malformation of the thigh in a 2-year-old girl. (a) Composite longitudinal US shows an elongated subcutaneous cystic mass with a few thick septations. (b) Color Doppler interrogation shows it is essentially avascular
The size of the cysts determines the appearance of the lesion at MRI. Lesions with microscopic cysts appear solid, with low signal on T1, high signal on T2, and either diffuse or complete absence of enhancement [21]. Macrocystic lesions have a more typical appearance, with multiple well-defined cysts that have low signal on T1 (unless there has been previous hemorrhage or infection) and high signal on T2 (Fig. 22.8). There may be fluid-fluid levels (Fig. 22.9). Although the septa may enhance, the cystic components do not enhance unless there has been prior treatment [21]. Like most vascular lesions, lymphatic malformations do not respect fascial planes and extend into multiple different tissue types (Fig. 22.10).
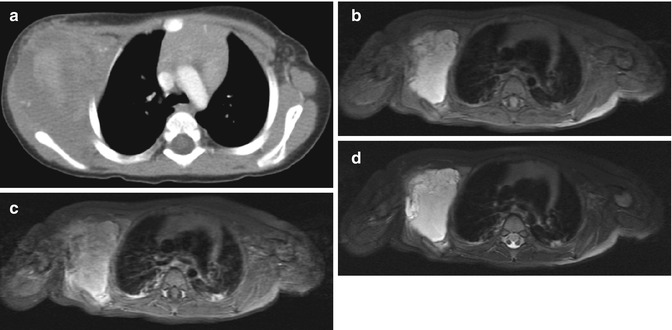
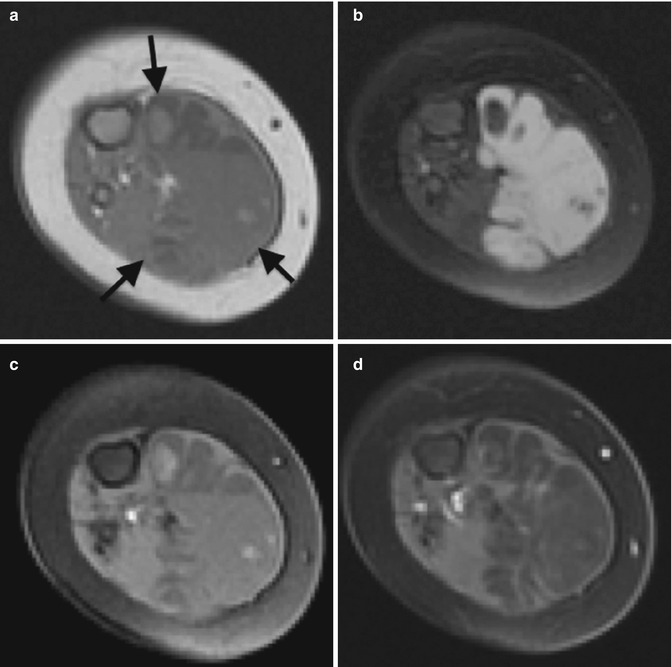
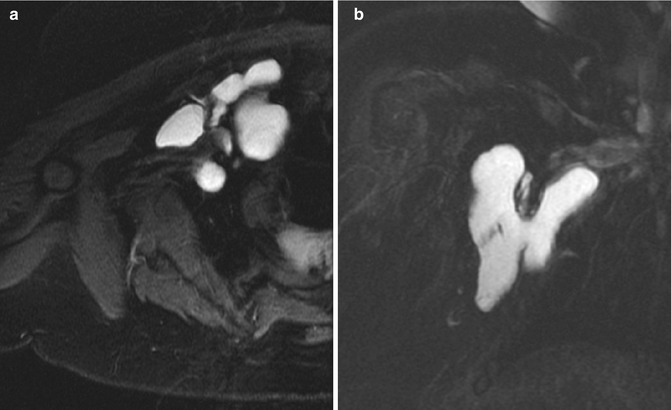

Fig. 22.8
Lymphatic malformation in the axilla of a 13-month-old boy. (a) Axial CT shows a nonspecific lobulated soft tissue mass. Axial T1-W FS (b) images demonstrate homogenous hyperintensity, indicating protein or hemorrhage. (c) Axial T1-W FS gadolinium-enhanced image shows no intralesional enhancement. (d) T2-W FS image shows moderately increased signal, consistent with complex fluid. Focal hypointensity likely represents hemosiderin

Fig. 22.9
Lymphatic malformation of the calf in a 5-month-old girl. (a) Axial T1-W image shows a fairly homogeneous mass in the medial head of the right gastrocnemius muscle (arrows), predominantly isointense to muscle, with several fluid-fluid levels and a few septations. Scattered areas of hyperintensity are probably fat. (b) Axial T2-W FS image shows a diffusely hyperintense mass with a few thin septations and a rounded area of decreased signal (likely hemorrhage). Axial T1-W FS (c) and T1-W FS gadolinium-enhanced (d) images show fluid-fluid levels and septal enhancement

Fig. 22.10
Infiltrative lymphatic malformation in the axilla of a 4-year-old girl. Axial (a) and coronal (b) T2-W FS images show a multiloculated bilobed mass that wraps around soft tissue structures
Syndromes Associated with Vascular Malformations
Klippel–Trenaunay–Weber syndrome (Fig. 22.11) consists of the triad of cutaneous hemangioma (manifesting as a port-wine stain), overgrowth of a limb or part of a limb, and varicose veins [27]. When the lesion is associated with an AVF, it is known as Parkes Weber syndrome. Both lesions usually demonstrate bony overgrowth, with increased fat and atrophy of the musculature. There is often associated fibromuscular dysplasia.
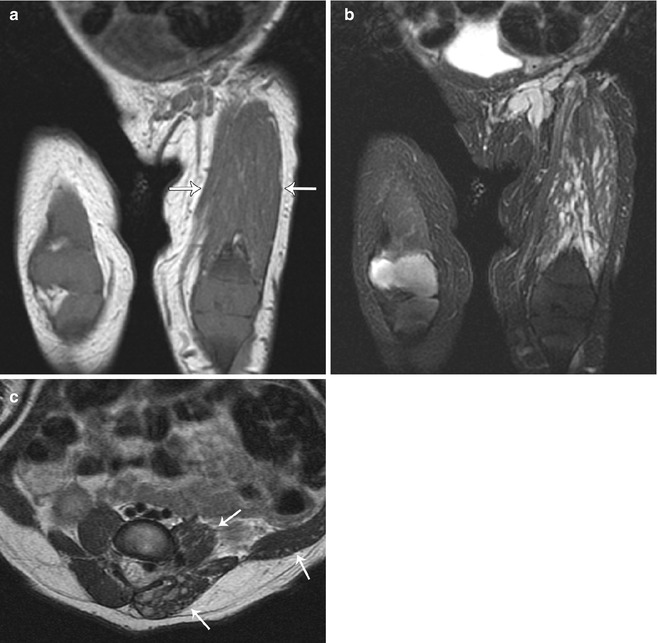

Fig. 22.11
Klippel-Trenaunay-Weber syndrome in a newborn. (a) Coronal T1-W image shows an isointense lymphaticovenous malformation insinuated within atrophic musculature (arrows). (b) Coronal T2-W FS image shows the mass extends to the inguinal area. (c) Axial T2-W image shows hyperintense cystic infiltration of the left erector spinae and other musculature (arrows), with enlargement of spinal veins (Reprinted from Stein-Wexler [98], copyright 2009, with permission from Elsevier)
Maffucci syndrome consists of multiple enchondromas and multiple soft tissue venous malformations – the characteristic phleboliths and enchondromas clinch the diagnosis on radiographs [27]. The enchondromas eventually transform into chondrosarcomas in 15–20% of cases, and the venous malformations transform into vascular sarcoma in 3–5 % of cases [27].
Blue rubber bleb nevus syndrome causes multiple cutaneous vascular lesions and venous malformations of the gastrointestinal tract.
Osler–Weber–Rendu syndrome is a systemic fibrovascular dysplasia that causes AVMs in multiple organs as well as in the extremities, along with aneurysms and telangectasia [27].
Kaposiform Hemangioendothelioma
This rare tumor is intermediate in the continuum between infantile hemangioma and angiosarcoma – more aggressive than a hemangioma, but not malignant [10]. Its clinical features resemble those of a proliferative infantile hemangioma, but it does not regress and responds poorly to standard treatments for infantile hemangiomas [11, 28]. It usually requires surgical excision with or without preoperative embolization [10]. The histologic appearance of this densely cellular tumor resembles Kaposi sarcoma, but kaposiform hemangioendothelioma is not associated with human herpes virus-8 infection [15]. Immunohistochemical stains can confirm the diagnosis [10].
Imaging
On US, kaposiform hemangioendothelioma appears as an infiltrative mass with variable echogenicity and both high- and low-resistance waveforms on color Doppler imaging [15]. Although quite vascular, it is typically less vascular than hemangioma [17]. The MRI appearance is nonspecific, with heterogeneous signal on T1, generally high signal on T2, and multiple surrounding vessels. There is usually subcutaneous stranding on T2-W sequences. The tumor enhances intensely. Unlike the infantile hemangioma, kaposiform hemangioendothelioma usually involves multiple tissue planes, causes skin thickening, and has abundant surrounding edema. There are fewer superficial feeding and draining vessels than with an infantile hemangioma, and adjacent bone destruction is more common [23].
Hematoma (Box 22.4)
Box 22.4: Hematoma
May present as palpable mass with no recollection of antecedent trauma. |
Even with history of trauma, evaluate an intramuscular hematoma with contrast-enhanced MRI to exclude underlying hemorrhagic tumor. |
Signal progression in soft tissue hematomas differs from CNS. |
Children are very active but relatively uncoordinated and therefore suffer a lot of bumps, bruises, and falls. Although most of these insults cause no appreciable damage, some result in soft tissue hematomas. The acute but transient discolored, palpable mass with overlying bruising is usually managed without imaging, but occasionally a child presents with questionable history or indeed years after the initial trauma with no recollection of a specific injury. Histology reveals a mass composed of blood clot, fibrin, and amorphous debris in a central cavity. There is a fibrous pseudocapsule that may contain foreign body giant cells, cellular scarring, xanthogranulomatous inflammation, cholesterol clefts, and possibly dystrophic calcifications.
Treatment usually consists of watchful waiting, though resection is sometimes performed when imaging findings are nonspecific or if the mass fails to resolve.
Imaging
Radiographs demonstrate nonspecific swelling (Fig. 22.12). If the hematoma has liquefied, a fluid-fluid level may be evident.
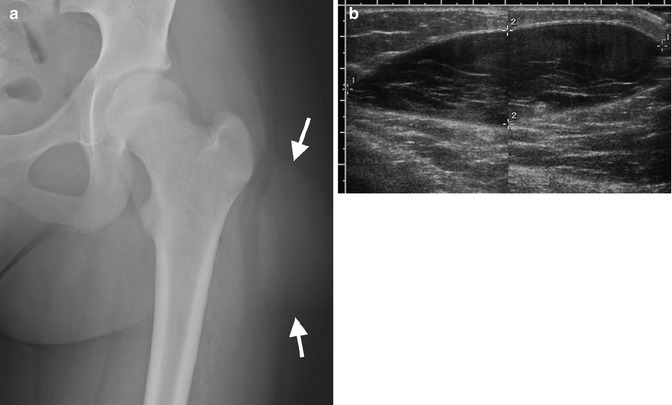

Fig. 22.12
Hematoma in a 13-year-old girl with a painful hip mass after trauma. (a) There is a lentiform soft tissue mass (arrows). (b) Composite US image shows a well-circumscribed, hypoechoic, subcutaneous lentiform mass, with internal septations. Color Doppler interrogation demonstrated no blood flow (not shown)
US shows a heterogeneous mass with either well-defined or irregular margins. Acute hematomas appear hyperechoic, whereas in later stages they appear hypoechoic [29]. Furthermore, clotted blood appears more echogenic when evaluated with a higher frequency transducer. There may be internal septations. If the hematoma is associated with a muscle tear, dynamic imaging with passive flexion and extension may show retraction of muscle with increased size of the lesion [29]. There should be no internal Doppler signal in a benign hematoma, although there may be increased flow around it; vascular flow within a hematoma suggests there is an underlying lesion.
The CT appearance depends on the lesion’s age. Acute blood is hyperdense, but subacute hematomas demonstrate mixed density, reflecting clot lysis. The rim may enhance after contrast administration [30]. Chronic hematomas are well-defined, homogeneous, and of water density. The fibrous pseudocapsule of a chronic hematoma may calcify.
The MRI appearance is variable and changes as lesions evolve. It is important to recognize that the absence of a blood-brain barrier in the soft tissues causes hemorrhage to behave differently there than in the brain. Blood products degrade more rapidly, and signal intensity progresses differently [31]. When intramuscular hematomas were experimentally induced in rats, the low T2 signal ascribed to intracellular deoxyhemoglobin in the acute and subacute phases of brain hemorrhage never materialized, perhaps because blood flow in skeletal muscle allowed red blood cells to break down more rapidly than within the brain. However, at late stages hypointense hemosiderin did develop [31].
The internal signal of a hematoma may be heterogeneous, reflecting blood in various stages of evolution. Acute blood products tend to be bright on T1-W images (Fig. 22.13). In the acute and subacute stages, edema may surround the hematoma, but this resolves as the hematoma matures (Fig. 22.14). As early as 4 days after injury, a hypointense rim may be present at T2-W images, although no hemosiderin is present at histology; this may result from a boundary effect between two areas with different magnetic susceptibility [31]. The chronic hematoma often has a hypointense hemosiderin rim that may enhance. A mass with signal characteristics consistent with blood products most likely represents a benign hematoma, but short-term follow-up with contrast-enhanced MRI may be warranted to exclude an underlying neoplasm.
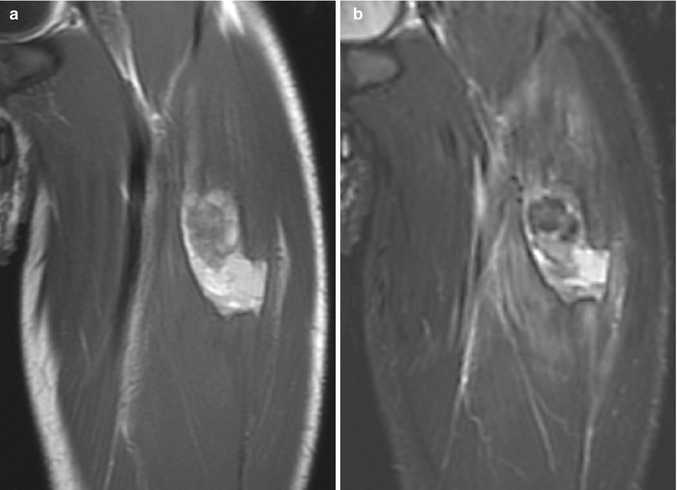
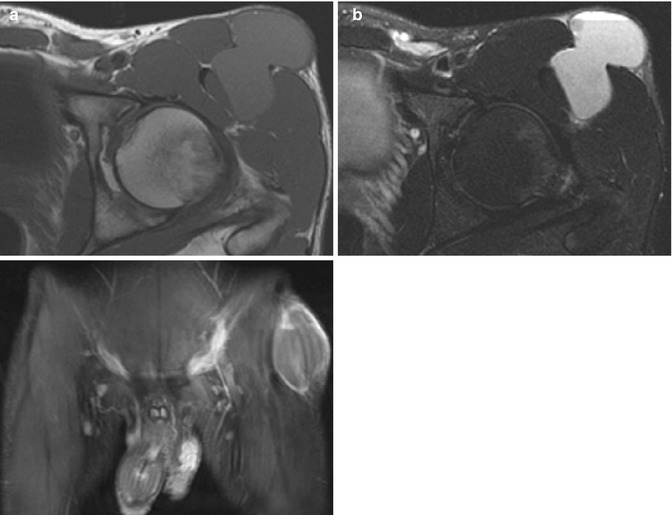

Fig. 22.13
Hematoma in a 14-year-old boy with sudden thigh pain while running. (a) Coronal T1-W image shows a moderately heterogenous. Mostly hyperintense mass within the rectus femoris muscle, with a thin hypointense rim. (b) Coronal T2-W FS image shows heterogenous, predominantly hyperintense mass with surrounding intramuscular edema

Fig. 22.14
Hematoma in a 15-year-old boy with chronic hip pain. (a) Axial T1-W image shows a bilobed, homogeneous, slightly hyperintense mass extending from intramuscular fascia to subcutaneous adipose tissue. (b) Axial T2-W FS image shows a fluid-fluid level and no surrounding edema. (c) Coronal T1-W FS gadolinium-enhanced image shows only a thin enhancing rim
Differential diagnosis is broad and includes abscess and a wide variety of sarcomas.
1.2 Fibrous Lesions
Desmoid Tumor
Also known as aggressive fibromatosis and as desmoplastic fibroma when it arises in or extends into bones, this non-metastasizing and histologically benign but locally aggressive tumor consists of intertwining fibroblasts and myofibroblasts [32, 33]. It arises most often in muscles in the extremities. Although cases are most common in the third decade, up to 30 % occur before age 20 (mean age 13 years) [32]. Predisposing conditions include antecedent trauma to the area, radiation, and, in between 1 and 2 % of cases, Gardner syndrome [32].
Clinical presentation includes pain, mass, and (if the tumor crosses a joint) limited mobility. Interlacing bundles of spindle cells with entrapped skeletal muscle and surrounding soft tissue on a background of abundant collagen are apparent at histology [32]. If there are nuclear atypia and multiple mitotic figures, the pathologist is more likely to render a diagnosis of sarcoma. The tumor has infiltrative borders and is usually tan white, with a whorled appearance and firm texture.
Treatment is difficult, as the tumor invariably extends beyond the margins evident at imaging and palpable at surgery, so despite aggressive, wide resection about 60 % of tumors recur [34]. Tumors that involve the neurovascular bundle and/or bone are more difficult to resect completely and consequently have a higher risk of recurrence [33]. Treatment often includes radiation and chemotherapy, though radiation occasionally induces additional desmoid tumors in the adjacent tissue [33]. Early experience suggests that cryoablation of desmoid tumors that do not involve vital structures provides long-term disease-free response in adults [35]; this may warrant further investigation in children.
Imaging
US appearance is variable and nonspecific [34]. At CT this tumor is similarly variable, but tends to be isodense to skeletal muscle [34]. There is no consistent relationship between CT appearance of the tumor and its histology. Intravenous contrast helps evaluate the relationship between the tumor and vessels.
MRI appearance (Fig. 22.15) varies with the extent of cellular, collagen, and myxoid material, though the tumor tends to be iso- to hyperintense to muscle on T1 and heterogeneously hyperintense to muscle on T2 [33]. It usually demonstrates avid enhancement. Linear and curvilinear strands of non-enhancing low-signal-intensity tissue that cross the tumor correspond to strands of collagen and may suggest the diagnosis [4]. Even if the margins of the tumor appear well circumscribed, there is usually microinvasion beyond the pseudocapsule at surgery. It is therefore essential to comment on adjacent important structures, such as the neurovascular bundle and joints. Features that have been reported to differentiate desmoid tumors from sarcomas include an infiltrative growth pattern, crossing of fascial planes, and lack of central necrosis even in very large lesions [36].
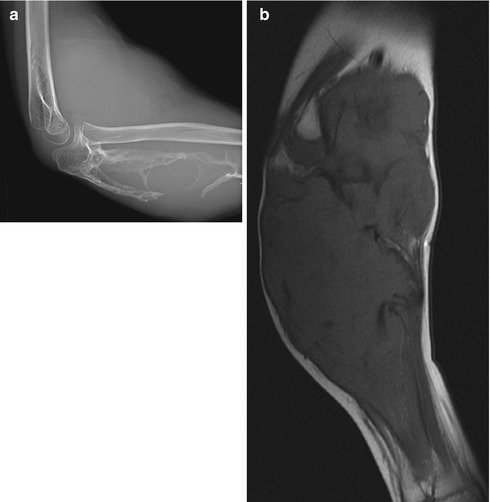
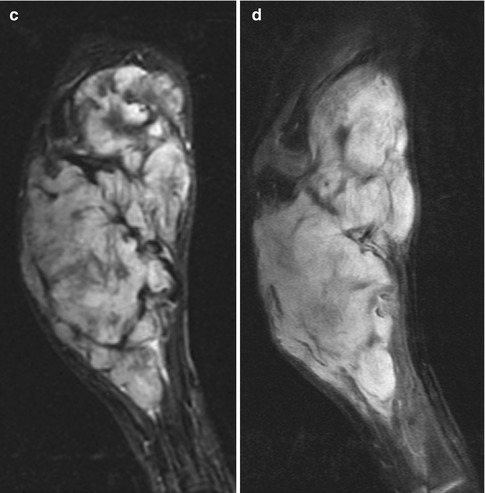


Fig. 22.15
Desmoid in a 9-year-old boy with an enlarging forearm mass. (a) A large mass expands and destroys the ulna. (b) Coronal T1-W image shows hypointense fibrous strands traversing a lobulated mass that is slightly hyperintense to skeletal muscle. (c) Coronal short tau inversion recovery (STIR) image shows it is heterogeneous, with hypointense strands. (d) Coronal T1-W FS gadolinium-enhanced image shows homogeneous enhancement (except for the fibrous strands)
Infantile Myofibromatosis
The nodules of infantile myofibromatosis usually present in the neonatal period and are rare after age 2 years. They are composed of spindle cells, with features of fibroblasts and smooth muscle. Rarely unifocal, multiple soft tissue nodules in the subcutaneous soft tissues of the head, neck, or trunk are typical. Occasionally the skeleton, gastrointestinal tract, heart, or lungs are involved. Spontaneous regression occurs in 30 %, although if there is visceral involvement this disease is lethal in 70 % [37]. Multifocality is more likely in girls.
Imaging
These lesions are round and can be well circumscribed or ill defined. Signal intensity varies, but they are typically isointense to muscle on T1-W imaging, although the center can be mildly hyperintense (Fig. 22.16). They are usually uniformly hyperintense on T2-W sequences. After administration of gadolinium, a target appearance is common, with lack of central enhancement but with intense enhancement in the periphery [21].
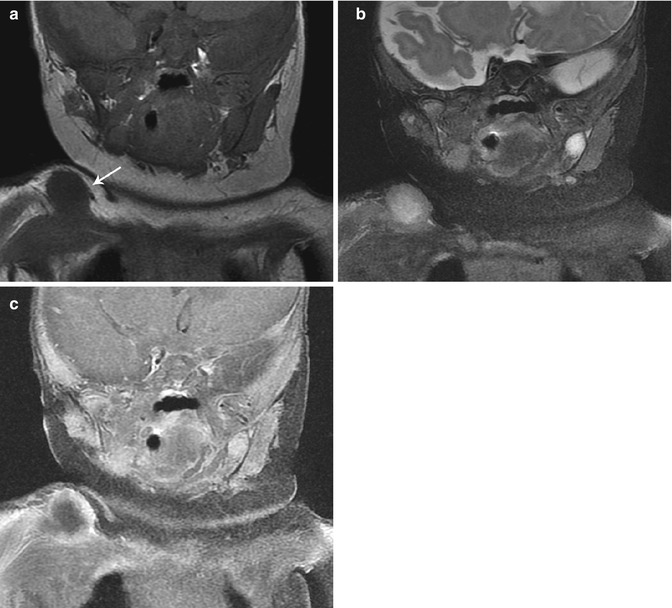

Fig. 22.16
Infantile myofibromatosis of the shoulder. (a) T1-W coronal image shows a rounded, well-defined, slightly hypointense right shoulder mass (arrow). The center is hyperintense on T2-W MRI (b), and there is marginal enhancement, resulting in a target appearance (c, T1-W FS gadolinium-enhanced) (Reprinted from Stein-Wexler [98], copyright 2009, with permission from Elsevier)
Popliteal Cyst
Two percent of asymptomatic children and 6 % of children referred for MRI due to knee pain demonstrate a popliteal cyst at imaging [38]. Cysts are more common in boys and are diagnosed at an average age of 8 years [39].
Imaging
On US, CT, and MRI, popliteal cysts present as well-delineated lesions extending through the space between the tendons of the medial head of the gastrocnemius muscle and the semimembranosus tendon (Fig. 22.17). Popliteal cysts in adults are almost always accompanied with joint pathology, but only 33 % of a series of patients referred from a pediatric rheumatologist had a knee-related risk factor for developing popliteal cysts (risk factors included juvenile idiopathic arthritis and Lyme arthritis) [40]. Other causes include unclassified oligoarthritis, gonoarthritis, trauma, benign joint hypermobility syndrome, hypophosphatasia, and a remote history of trauma [39]. However, any process within the joint that leads to an effusion can result in a popliteal cyst, including osseous lesions (Fig. 22.18). Some pediatric popliteal cysts may develop when seated children dangle their legs over the edge of a chair, irritating a weak portion of the posterior joint capsule; the presence of bursal thickening, as well as acute and chronic inflammation, supports this theory [41].
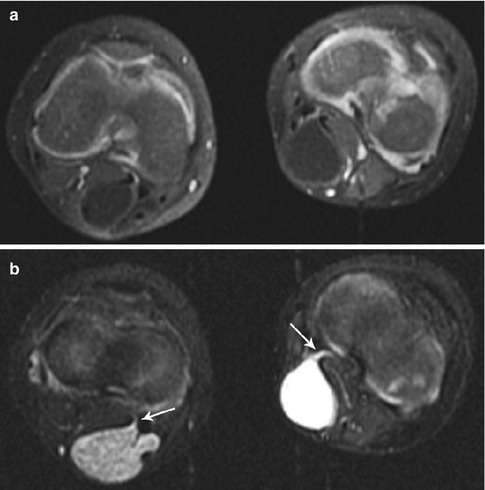
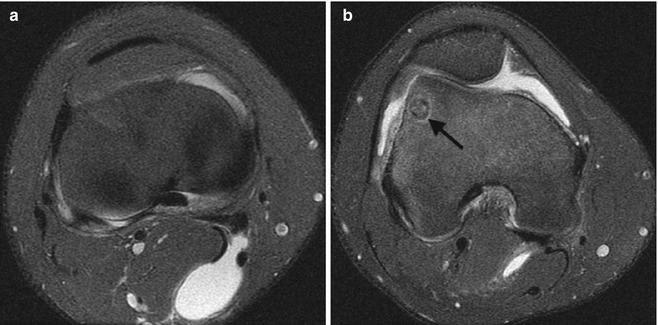

Fig. 22.17
Bilateral popliteal cysts in a 3-year-old girl. (a) Axial T1-W FS gadolinium-enhanced image shows bilateral cystic structures in the popliteal fossae. (b) Axial STIR image shows hemorrhage in the lesion on the right and simple fluid in that on the left. Both have tails (arrows) between the medial head of the gastrocnemius tendon and that of the semitendinosus, directed to the joint capsule. Both resolved spontaneously (Reprinted from Stein-Wexler [98], copyright 2009, with permission from Elsevier)

Fig. 22.18
Popliteal cyst in a 15-year-old boy with knee pain. (a) Axial PD FS images demonstrate (a) a cyst extending between the tendons of the medial head of the gastrocnemius and the semimembranosus tendon and (b) a joint effusion due to an osteoid osteoma (arrow)
Popliteal cysts are an important entity to keep in mind solely to keep from confusing them with significant pathology. Usually asymptomatic, they require no specific treatment other than treating the underlying predisposing condition, if any.
1.3 Fatty Lesions
Lipoblastoma/Lipoblastomatosis
Lipoblastoma and its infiltrative form, lipoblastomatosis, are rare, benign tumors of embryonal white fat that usually occur before the age of 4 years. They are approximately three times more common in boys [42]. The presenting symptoms relate to the size and location of the mass, but the mass itself is not painful [42]. Left alone, they mature into a benign lipoma [43].
Histologic evaluation reveals a spectrum of fat cells in various stages of maturation, ranging from lipoblasts to mature adipocytes, embedded in a variable amount of myxoid stroma; the myxoid component is anecdotally more prominent in very young patients [42]. There is a rich capillary network. Grossly, lipoblastoma ranges from red to pink to bright yellow and is composed of lobulated masses of fat with fibrous septations. Lipoblastomatosis has a similar appearance, but infiltrates the muscle and surrounding tissues.
The treatment for both lipoblastoma and lipoblastomatosis is gross total surgical resection. Lipoblastomatosis, an infiltrative lesion more difficult to completely resect, is more likely to recur.
Imaging
Imaging reflects the proportion of fat to myxoid material. Radiographs can demonstrate a lesion more lucent than muscle. US, CT, and MRI demonstrate a mass with echogenicity, density, and signal intensity identical to that of the adjacent subcutaneous fat (Fig. 22.19). The rich capillary network in some tumors can result in intense enhancement [44].
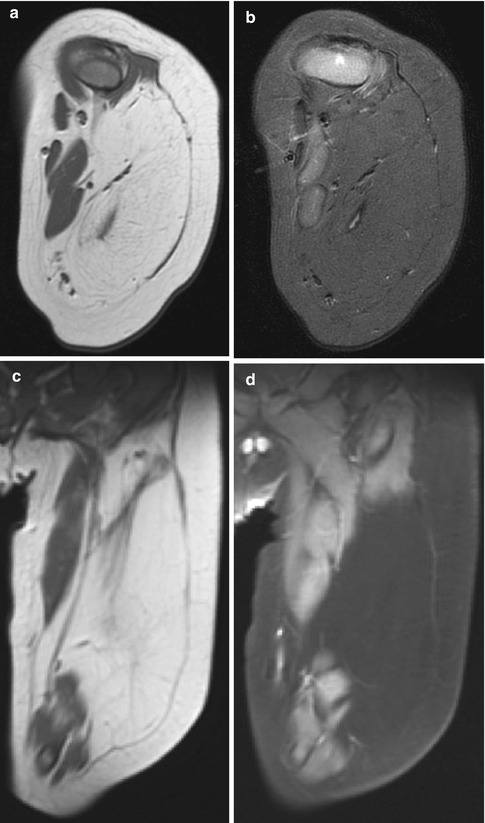

Fig. 22.19
Lipoblastomatosis of the leg in a 7-month-old boy. (a) Axial T1-W image shows a large, infiltrative mass with signal intensity identical to that of fat. (b) Signal intensity is completely suppressed on axial T1-W FS image. (c) Coronal T1-W and corresponding (d) T1-W FS gadolinium-enhanced images show the mass does not enhance
Differential Diagnosis
A predominantly fatty lesion in a child below age 10 is almost always lipoblastoma [42] and much less likely a hibernoma or fibrous hamartoma of infancy, which can occasionally contain fat. In patients over age 10, angiolipoma and lipoma are additional considerations [44]. An intensely enhancing lipoblastoma resembles a myxoid liposarcoma, but this lesion is rare in children [42].
Other Fatty Tumors: Lipoma and Hibernoma
Lipomas in older children and teens resemble those in adults, consisting of a well-circumscribed, homogeneous mass with signal, density, and echogenicity identical to subcutaneous fat [17, 42].
Hibernomas are composed of brown fat, which has an unusually rich blood supply and many mitochondria. They are relatively rare, and only 5 % occur in children [45]. They present as a soft, mobile mass, most common in the thigh. The hibernoma may feel warm relative to surrounding tissues [42]. Surgical excision is definitive, with no reports of metastases or recurrence.
Imaging
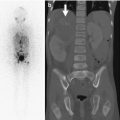
The hibernoma appears lucent on radiographs. US demonstrates an echogenic, well-circumscribed mass that may have increased flow on Doppler imaging [42]. CT and MR appearance varies with the myxoid content of the tumor but is usually similar although not identical to fat. Enhancement is intense, which might raise concern for liposarcoma, but characteristic branching and serpentine high-flow vessels in and around the mass with additional low-flow vessels (never seen in liposarcoma) should suggest the diagnosis [42].
Bone scan with Tc-99m MDP demonstrates radiotracer uptake on flow and blood pool images. Positron emission tomography (PET) scan demonstrates intense fluorodeoxyglucose (FDG) uptake due to the lesion’s high glucose metabolism [42].
Fat Necrosis
Trauma, injections, cold exposure, sickle cell disease, and vasculitis can injure the subcutaneous fat, usually in the hips, thighs, back, shoulders, and cheeks – areas with protuberant bones [17]. Neonates are particularly prone to fat necrosis, due to obstetrical injury, hypoxia, and hypothermia [46]. On histology, the lesion consists of necrotic adipose tissue with either admixed granulation tissue and lymphocytes or intense vasculitis [46, 47].
Fat necrosis presents as a firm mass in the subcutaneous tissues with possible overlying erythema; it may be mildly tender or asymptomatic [48]. The patient often does not recall antecedent trauma. A focus of fat necrosis usually enlarges slowly over weeks to months and then resolves over additional weeks to months, possibly leaving a subtle depression in the overlying skin. An important side effect of fat necrosis is hypercalcemia, which may develop up to 6 months after the insult. The current theory is that excess production of vitamin D by histiocytes infiltrating the necrotic fat causes increased uptake of dietary calcium; the serum calcium levels in neonates with fat necrosis should therefore be monitored for 6 months [46]. Treatment is either watchful waiting or, in equivocal cases, surgical resection.
Imaging (Fig. 22.20)
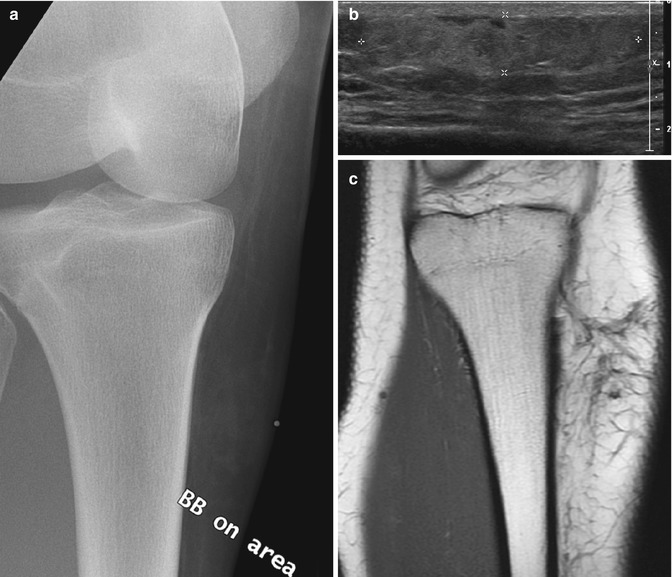
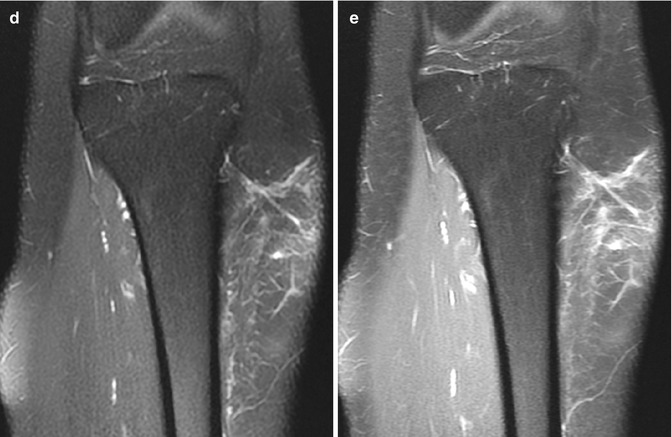
Fig. 22.20
Fat necrosis in a 16-year-old girl with a palpable, tender mass 2 weeks after being kicked in the shin. (a) There is hazy stranding in the subcutaneous fat. (b) Longitudinal US shows a hyperechoic, ill-defined subcutaneous mass (almost avascular at color Doppler (not shown)). (c) Coronal T1-W MRI shows linear hypointense stranding, hyperintense at (d) T2-W FS image. (e) Coronal T1-W FS gadolinium-enhanced image shows the strands enhance slightly, and there is no mass
Radiographs demonstrate subtle stranding of the subcutaneous fat. The appearance varies at US, but all lesions are located in the subcutaneous fat. Fat necrosis can appear as a hyperechoic nodule in the subcutaneous fat, with poorly defined margins and very little vascularity on Doppler imaging [17]. Other appearances include well-defined nodules that are isoechoic to surrounding fat, with a hypoechoic rim, and heterogeneously hyperechoic and ill-defined lesions with increased flow on Doppler imaging [47].
CT may demonstrate a discrete soft tissue density nodule in the subcutaneous fat or diffuse stranding of the subcutaneous fat without extension beyond the superficial fascia [46]. On PET scans [49], these nodules may be FDG avid [47].
MRI appearance varies as well. There may be intermediate signal on T1-W and T2-W sequences, with diffuse contrast enhancement, or there may be heterogeneous signal on both sequences, with intense ring enhancement [47]. Linear signal intensity in the subcutaneous fat with heterogeneous T2 signal and no focal mass has also been reported [17]. The lack of a focal mass is reported to be the most distinguishing imaging feature [50].
Differential Diagnosis
This includes cellulitis and, for heterogeneous, ill-defined lesions, soft tissue sarcomas and aggressive fibromatosis.
1.4 Neurofibroma (Box 22.5)
Box 22.5: Neurofibroma
90 % are NOT associated with neurofibromatosis I |
Localized: fusiform mass with true capsule, around nerve |
Split fat sign on MRI/CT: rim of fat around small neurofibroma |
Target sign: hypointense center, T2 hyperintense surrounding myxomatous tissue |
Diffuse: infiltrate subcutaneous fat and perineural soft tissue, plaque like |
Plexiform: infiltrative, nodular ropelike mass along nerves |
Malignant degeneration (5–10 % of plexiform neurofibromas): |
More likely if growth, pain, neurological symptoms |
A neurofibroma is a benign proliferation of the connective tissue of the nerve sheath [51]. Neurofibromas may grow in three different patterns – localized, diffuse, and plexiform.
Localized neurofibromas are most common, representing 90 all percent of neurofibromas. These tumors involve superficial cutaneous nerves. Diffuse neurofibromas primarily occur in children and young adults, most often in the head and neck [52]. Even though neurofibromas are classically associated with neurofibromatosis type 1 (NF1), 90 % of all neurofibromas are solitary lesions not associated with the syndrome [52, 53].
Localized neurofibromas present as fusiform soft tissue masses. When they involve a large peripheral nerve, they may be deep seated. On imaging and at surgery they are well circumscribed and have a true capsule. When they involve a small peripheral nerve, they maintain a true capsule even if they extend beyond the borders of the nerve [52]. Diffuse neurofibromas, in contrast, infiltrate into the subcutaneous fat and other soft tissues around the nerve, resulting in a poorly defined lesion that presents as a plaque-like elevation of the overlying skin and subcutaneous soft tissues.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree