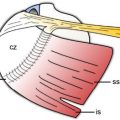
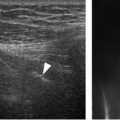
Figure 4-2. Minimum protocol to evaluate the SSB. [A] Positioning of the probe for long-axis view over the supraspinatus tendon (see figure 1-11A for arm positioning). [B] Corresponding 12-5 MHz US image shows the normal SSB bursa as a hypoechoic structure (arrowheads) separated from the deltoid (delt) and supraspinatus (supra) by a thin hyperechoic line representing the peribursal fat and adjacent fascia. [C] Positioning of the probe for long-axis view just distal to the supraspinatus tendon. [D] Corresponding 12-5 MHz US image shows the normal SSB. [E] Positioning of the probe for short-axis view over the supraspinatus. [F] Corresponding 12-5 MHz US image shows the normal SSB. [G] Positioning of the probe for long-axis view over the subscapularis tendon with the shoulder in external rotation. [H] Corresponding 12-5 MHz US image shows the normal SSB. From each image the SSB can be measured perpendicular to the humeral head cortex excluding the echogenic peribursal fat. Additional images may also be obtained to assess the maximal thickness of the bursa. Sub= subscapularis.
4.1.2. Spectrum of Findings
At US, an abnormal bursa may show (1) fluid distension, (2) synovial proliferation, and/or (3) thickening of the bursal walls. In any case, the magnitude of pathological findings does not correlate with the magnitude of the symptoms.
The diagnosis of subacromial-subdeltoid bursitis based on incompressible hypoechoic thickening of the bursal walls is debated, and there is no consensus agreement on the diagnostic criteria to define pathology.27 In general lines, we consider 2 mm as a reliable cutoff to indicate thickening of the bursal walls. The measurement should not include the peribursal echogenic fat (figure 4-3). Lower thresholds for diagnosis require contralateral asymmetry and correlation with symptoms (figure 4-4). Care must be taken not to confuse the SSB with the deep portion of the normal hypoechoic deltoid muscle surrounded by echogenic fascia (figure 4-5). Also, proximal musculotendinous junction of the supraspinatus may mimic pathological bursa to the inexperienced examiner (figure 4-6). Subacromial-subdeltoid bursitis often coexists with supraspinatus tendinopathy and it may be difficult to sonographically differentiate the hypoechoic bursa from the underlying hypoechoic tendon. Dynamic evaluation during shoulder abduction may help to differentiate between these two structures because it occasionally demonstrates contact between the coracoacromial ligament and the SSB. Nonetheless, as will be discussed later, contact between the coracoacromial ligament and the SSB is not specific to symptoms and can also be found in healthy volunteers.28 Focal or diffuse thickening of the superficial peribursal echogenic fat is occasionally depicted but its clinical relevance is largely unknown (figure 4-7). Theoretically, such areas can result from inflammatory reaction of the fat pad secondary to subacromial impingement. However, controlled studies are needed to correlate this imaging finding with surgical and clinical evaluation. As rotator cuff disease often parallels SSB pathology, asymptomatic bursitis may be as highly prevalent as asymptomatic rotator cuff disorders.
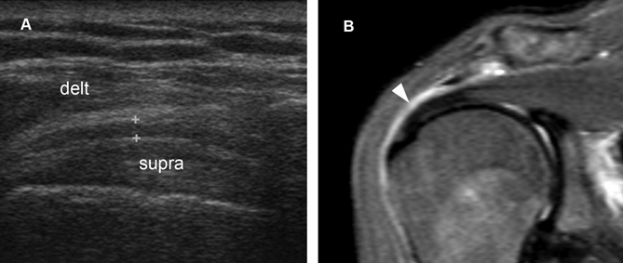
Figure 4-3. Subacromial-subdeltoid bursitis. [A] Coronal oblique 12-5 MHz US image shows hypoechoic thickening of the SSB (cursors). [B] Corresponding coronal oblique MRI confirms the diagnosis of bursitis (arrowhead). Delt= deltoid. Supra= supraspinatus.
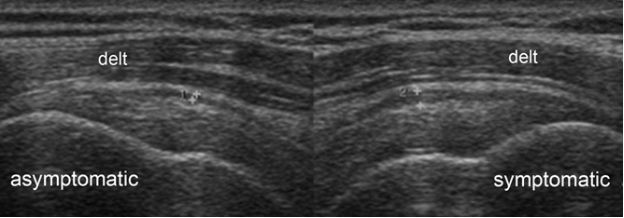
Figure 4-4. Subacromial-subdeltoid bursitis. Coronal oblique 12-5 MHz comparative US image shows asymmetric hypoechoic thickening of the SSB due to bursitis in the symptomatic side (cursors). Delt= deltoid.
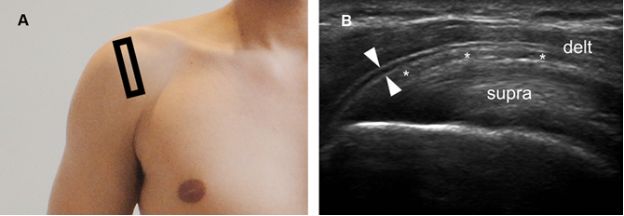
Figure 4-5. Pitfall. [A] Positioning of the probe. [B] Corresponding coronal oblique 12-5 MHz US image shows a fascial sheath of the deltoid muscle generating a pseudobursal appearance (arrowheads). The normal hypoechoic SSB is identified deep to the pseudobursa (asterisks). Delt= deltoid. Supra= supraspinatus.
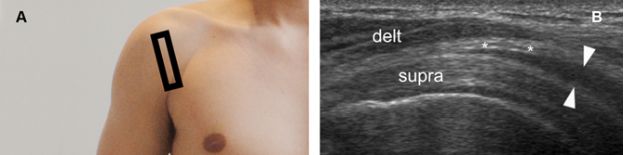
Figure 4-6. Pitfall. [A] Positioning of the probe. [B] Corresponding coronal oblique 12-5 MHz US image shows normal proximal hypoechoic muscle located at the musculotendinous junction of the supraspinatus generating a pseudobursal appearance (arrowheads). The normal hypoechoic SSB is identified superficial to the pseudobursa (asterisks). Delt= deltoid. Supra= supraspinatus.
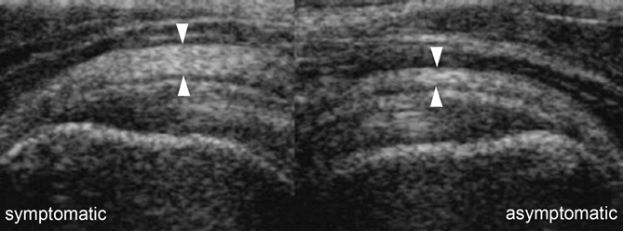
Figure 4-7. Diffuse thickening of the peribursal fat. Comparative coronal oblique 12-5 MHz US image shows diffuse thickening of the superficial peribursal echogenic fat in the symptomatic side (arrowheads). The SSB is normal.
The diagnosis of subacromial-subdeltoid bursitis based on fluid distension of the bursal cavity is more intuitive since sonographic detection of any amount of compressive fluid is considered abnormal (video 4-1). When the patient is standing or in sitting position, fluid tends to accumulate distally in the most dependent anterolateral aspect of the bursa (figure 4-8). Another dependent area is located anteriorly over the intertubercular groove, often included in the field of view when evaluating the long head of biceps brachii tendon for disease. Care should be taken not to apply excessive pressure with the probe because bursal fluid may become undetectable if displaced (figure 4-9). In long-standing cases, the bursa may develop loculi (figure 4-10; video 4-2) and septations (figure 4-11). Fluid distension of the SSB may also result from its communication with the glenohumeral joint via a full-thickness tear of rotator cuff tendons. In such cases, graded compression with the free hand of the examiner can reveal fluid passing from the SSB to the glenohumeral joint and vice versa during real time evaluation. Communication with the glenohumeral joint allows the SSB to serve as a reservoir for loose bodies (figure 4-12; video 4-3).
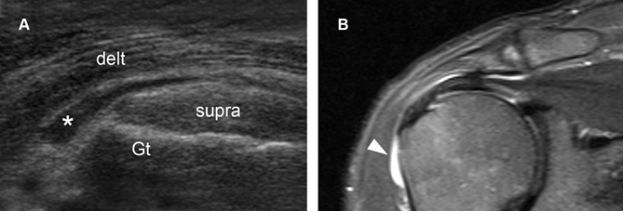
Figure 4-8. Subacromial-subdeltoid bursitis. [A] Coronal oblique 12-5 MHz US image shows fluid distension of the SSB (asterisk) extending anterolaterally beyond the greater tuberosity (Gt), the so called tear drop sign. [B] Corresponding coronal oblique STIR MRI confirms sonographic finding (arrowhead). Delt= deltoid. Supra= supraspinatus.
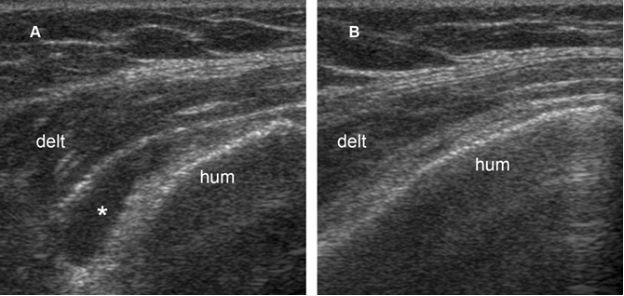
Figure 4-9. Subacromial-subdeltoid bursitis. [A] Coronal oblique 12-5 MHz US image shows fluid distension of the SSB (asterisk). [B] Excessive pressure with the probe displaces the fluid and collapses the bursa. Hum= humerus. Delt= deltoid.
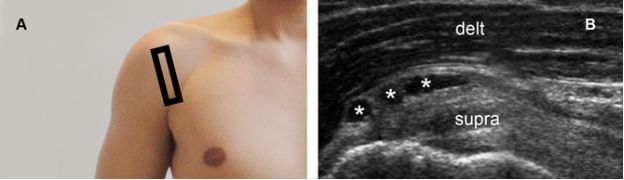
Figure 4-10. Subacromial-subdeltoid bursitis. [A] Positioning of the probe. [B] Corresponding 12-5 MHz US image depicts multilocular SSB (asterisks). Delt= deltoid. Supra= supraspinatus.
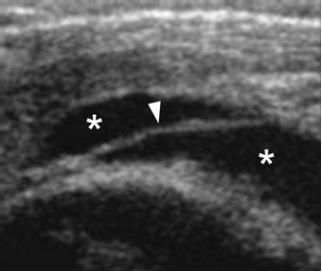
Figure 4-11. Subacromial-subdeltoid bursitis. Coronal oblique 12-5 MHz US image shows fluid distended SSB (asterisks) containing a thin septum (arrowhead).
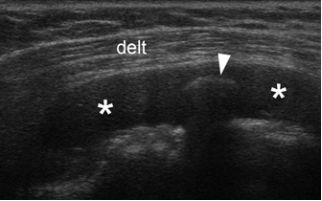
Figure 4-12. Loose body. Coronal oblique 12-5 MHz US image shows fluid distended bursa (asterisks) containing a calcific loose body (arrowhead). Delt= deltoid.
Complex hypo- or hyperechoic fluid may also be detected, but these characteristics do not accurately predict inflammatory, hemorrhagic or infectious process (figure 4-13). Synovial proliferation must always be included in the differential diagnosis of complex fluid, though both conditions can usually be confidentially differentiated at US because the former shows poor compressibility, lack of internal movements during graded compression, and occasional internal vascularization on Doppler interrogation. Synovial proliferation is especially common and can be massive in patients with systemic inflammatory arthropathies, mimicking an intrabursal mass (figure 4-14). Synovial chondromatosis should be considered in the differential diagnosis of intrabursal masses as it courses with a variable degree of synovial proliferation or hyperplasia. First described by Leannac in 1813,29 synovial chondromatosis can be differentiated into a primary and secondary form. The primary form represents an idiopathic benign neoplastic process affecting subintimal cartilage of a joint, tendon sheath, or bursa.30 In contrast, secondary chondromatosis does not show the cytogenetic aberrations observed in the primary form, probably represent metaplasia of synovial tissue into cartilaginous tissue, and occurs in association with preexisting traumatic, inflammatory, and degenerative conditions. In some cases, it may be difficult to differentiate primary from secondary synovial chondromatosis because long-standing primary synovial chondromatosis also predisposes to degenerative changes. Primary synovial chondromatosis is relatively rare and invariably presents as a monoarticular disease. Polyarticular involvement suggests the more common secondary form. Knees are the most commonly affected joints, possibly because of their abundance of synovial tissue. The extra-articular form is particularly rare and is frequently referred to as tenosynovial or bursal chondromatosis. Regardless of location and etiology, the resultant subintimal nodule of hyaline cartilage may detach from the synovium and produce chondral loose bodies within the joint, bursa, or tendon sheath. In primary synovial chondromatosis, the numerous chondral bodies are usually similar in size, which suggests a similar time frame origin. Conversely, chondral fragments generated by secondary synovial chondromatosis are fewer in number and more variable in size when compared with the fragments observed in primary disease. Because cartilage is nourished by absorption of nutrients and oxygen from the synovial fluid, detached chondral bodies may gradually increase in size. Fusion or coalescence of multiple chondral bodies may also occur, creating a soft tissue mass appearance.31 Most chondral bodies calcify over time, and a small proportion progress further and undergo ossification.32 Malignant transformation of primary synovial chondromatosis to chondrosarcoma occurs in 5% of cases and is suggested by multiple recurrences and marrow invasion depicted on MRI. In fact, the pathological appearance of primary synovial chondromatosis may simulate chondrosarcoma because of significant histologic atypia, and imaging correlation to localize the process as synovially based is vital for correct diagnosis.30 At US, synovial chondromatosis may present as a calcified heterogeneous avascular mass with variable shadowing (figures 4-15 and 4-16). Extrinsic erosion of bone may also be present. Extensive shadowing from calcified or ossified lesions may obscure the soft tissue mass and the multiplicity of the loose bodies. Dynamic evaluation is helpful to document change in position of unstable loose bodies. Treatment of primary disease is surgical synovectomy with removal of loose bodies. Recurrence rates are estimated between 3% and 23%33-35 and usually secondary to incomplete resection. The differential diagnosis of synovial chondromatosis includes pigmented villonodular synovitis, which represents an uncommon benign neoplastic process that affects the synovium. The extra-articular form of pigmented villonodular synovitis is even rarer and can involve tendon sheaths (pigmented vilonodular tenosynovitis) and bursae (pigmented villonodular bursitis).36,37 Histologically, the hypertrophic synovium is villous, nodular, or villonodular and contains variable amounts of hemosiderin.38 At US, pigmented villonodular bursitis usually appears as a fixed mural nodule (figure 4-17).39 MRI is helpful to diagnosis because it typically shows low signal foci on T2-weighted images and susceptibility artifact on gradient echo sequences due to the paramagnetic effect of hemosiderin-laden synovial tissue, which is usually very prominent in pigmented vilonodular bursitis.32
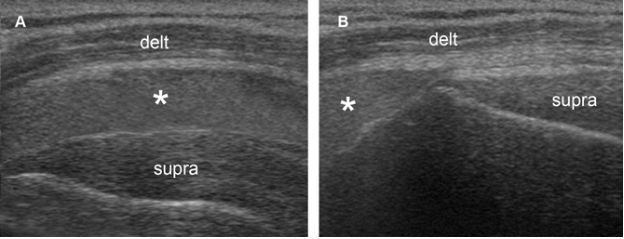
Figure 4-13. Subacromial-subdeltoid bursitis. [A] Coronal oblique 12-5 MHz US image shows echogenic fluid distending the SSB (asterisk). [B] Graded compression with the probe displaces the fluid. Delt= deltoid. Supra= supraspinatus.
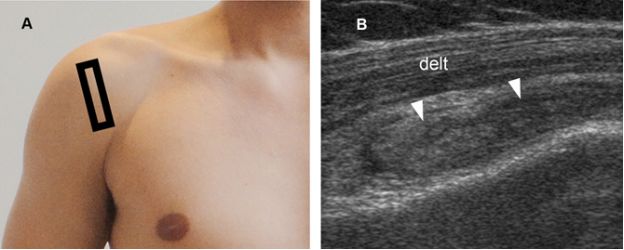
Figure 4-14. Subacromial-subdeltoid bursitis. [A] Positioning of the probe. [B] Corresponding coronal oblique 12-5 MHz US image shows synovial proliferation distending the SSB (arrowheads). Delt= deltoid.
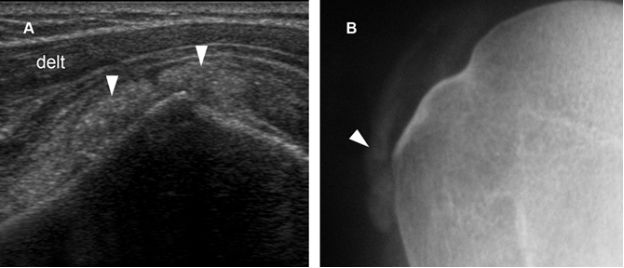
Figure 4-15. Synovial osteochondromatosis. [A] Coronal oblique 12-5 MHz US image shows SSB containing calcified synovial tissue with faint acoustic shadow (arrowheads). [B] Corresponding radiography confirms sonographic finding. Delt= deltoid.
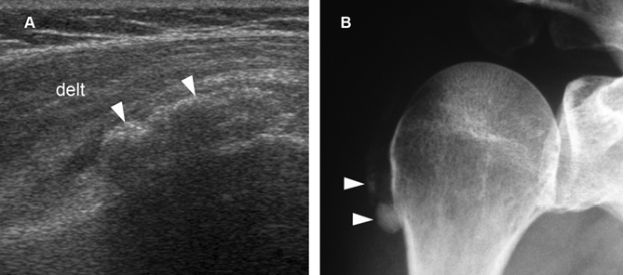
Figure 4-16. Synovial osteochondromatosis. [A] Coronal oblique 12-5 MHz US image shows a heterogeneous calcified mass containing multiple tiny hyperechoic foci (arrowheads). Acoustic shadowing is not depicted because there is no sufficient mineralization or endochondral bone formation. [B] Corresponding radiography confirms sonographic finding. Delt= deltoid.
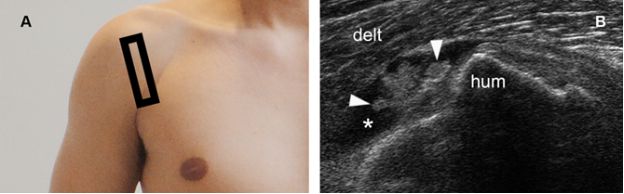
Figure 4-17. Pigmented villonodular synovitis. [A] Positioning of the probe. [B] Corresponding 12-5 MHz US image shows fluid distended bursa (asterisk) containing a vegetating lesion attached to the bursal wall (arrowheads). Delt= deltoid. Hum= humerus.
Apart from thickening of the bursal walls, synovial proliferation, and fluid distension of the bursa, rice bodies may also be detected. Rice bodies consist of concentrically laminated masses of fibrin and represent an end product of synovial proliferation, inflammation or degeneration40 There are many different theories about their pathogenesis, including bleeding within the bursa and fibrinous degeneration of the infarcted synovial tissue. Rice bodies are not exclusively found in chronic disorders and can occur early or late in the course of disease.41 They are usually present in large numbers, resemble rice grains (hence the name) and are depicted as isoechoic to hyperechoic nodules that may mimic complex fluid containing blood or debris (figure 4-18). Rice bodies can induce symptoms from chronic irritation that subside after effective removal by aspiration, lavage, or instillation of fibrinolytic agents.
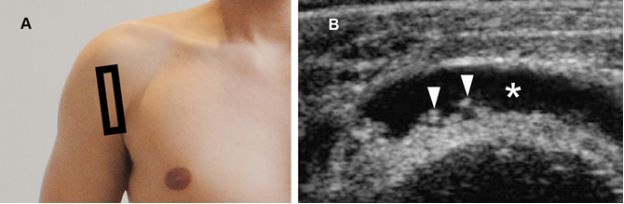
Figure 4-18. Rice bodies. [A] Positioning of the probe. [B] Corresponding 12-5 MHz US image shows fluid distended SSB (asterisk) containing multiple small rice bodies (arrowheads).
The Role of Doppler
Doppler evaluation may be useful to investigate SSB disorders because blood flow rates in normal bursae are relatively low and neovascularization suggests an active process that can correlate with symptoms.42 Adequate settings are essential to maximize low flow detection, and the color box must be sized to the area of interest to reduce background noise and optimize system resources (figure 4-19). Subacromial-subdeltoid bursitis may develop neovascularization via numerous mechanisms, including high concentrations of vascular endothelial growth factor and the combination of substance P and interleukin-1 (video 4-4).43
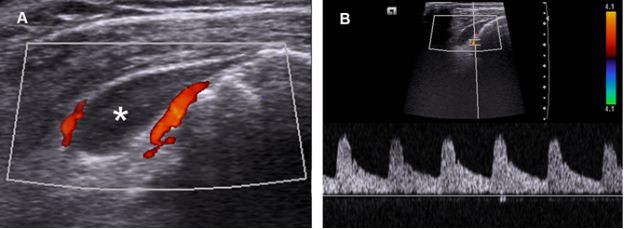
Figure 4-19. Subacromial-subdeltoid bursitis. [A] Coronal oblique 12-5 MHz Doppler US image shows fluid distension of the SSB extending anterolaterally beyond the greater tuberosity (asterisk). Note also increased peribursal vascularity. [B] Spectral analysis demonstrates arterial low-resistance Doppler waveform.
The Role of Dynamic Evaluation
The role of dynamic sonographic evaluation to detect subacromial impingement is not free of controversy. Although earlier studies have demonstrated bursal thickening following shoulder abduction in symptomatic shoulders,44-47 a more recent investigation found no significant difference in the degree of gathering of the SSB in impingement patients compared with healthy volunteers (figure 4-20).28 Anecdotal experience also suggests that slight contact between the coracoacromial arch and the SSB can occur in healthy individuals (video 4-5). However, significant contact (figure 4-21; video 4-6) or snapping (video 4-7) between these two structures are not common in the absence of symptoms and suggest clinically relevant subacromial impingement.
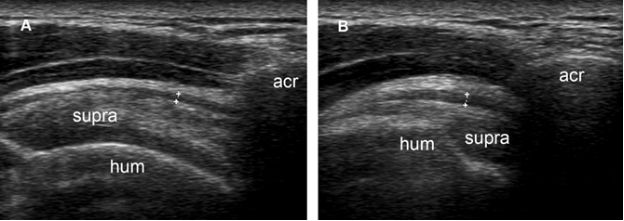
Figure 4-20. Normal gathering of the subacromial-subdeltoid bursa during shoulder abduction in a healthy volunteer. [A] Coronal oblique 12-5 MHz US image obtained during shoulder adduction shows normal subacromial-subdeltoid bursa (cursors). [B] Corresponding 12-5 MHz US image obtained during shoulder abduction shows gathering of the subacromial-subdeltoid bursa. Acr= acromion. Supra= supraspinatus. Hum= humerus.
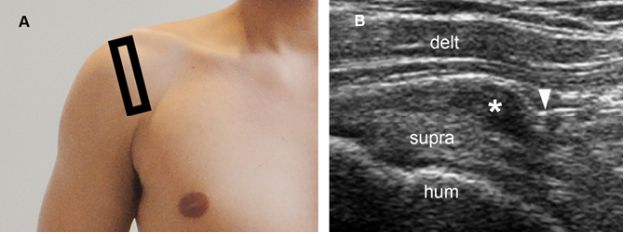
Figure 4-21. Clinically relevant subacromial impingement. [A] Positioning of the probe. [B] Corresponding 12-5 MHz US image obtained during shoulder abduction shows impingement of the coracoacromial ligament (arrowhead) over the subacromial bursa (asterisk). Supra= supraspinatus. Hum= humerus. Delt= deltoid.
4.1.3. Grading of Subacromial-Subdeltoid Bursitis
We generally do not report measurements of the bursal thickness because it has no proven influence on prognosis and may not correlate with the magnitude of bursal distension (figure 4-22). We also do not subjectively grade subacromial-subdeltoid bursitis, except for the very mild (e.g.: asymmetric incompressible hypoechoic thickening of the bursal walls < 2mm) or very severe cases (e.g.: fluid distension > 10mm in thickness). Although the amount of fluid does not correlate with symptoms, patients with more severe fluid distension typically show more advanced rotator cuff pathologies as an associated finding (figure 4-23).
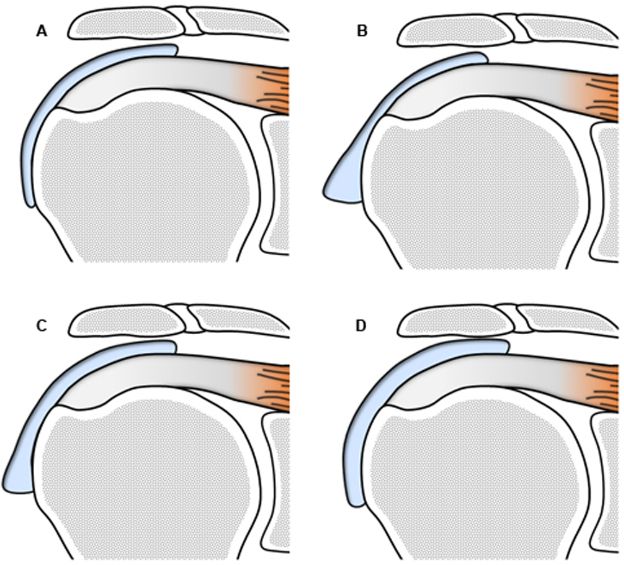
Figure 4-22. Inadequacy of numbers to follow-up of subacromial-subdeltoid bursitis. Maximal thickness of SSB estimated at [A] 2 mm, [B] 5 mm, [C] 4 mm, and [D] 3 mm. Hence, based on maximal thickness, the magnitude of bursal disease is estimated at A < D < C < B. Different results are obtained when considering bursal volume: A < B < C < D. It is inadequate to mathematize subacromial-subdeltoid bursitis to follow-up because it creates an artificial environment for comparison that may not correlate with the magnitude of bursal disease.
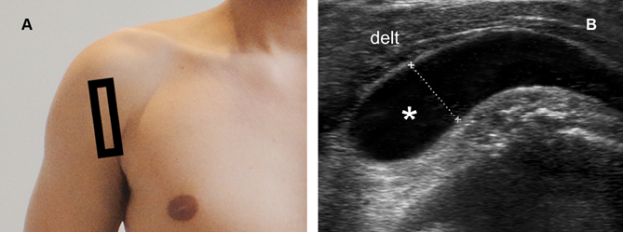
Figure 4-23. Severe subacromial-subdeltoid bursitis. [A] Positioning of the probe. [B] Corresponding 12-5 MHz US image shows fluid distension of the SSB (asterisk). Fluid thickness is estimated at 1.1 cm (cursors). Delt= deltoid.
4.1.4. Follow-up of Subacromial-Subdeltoid Bursitis
From the several presentations of subacromial-subdeltoid bursitis, only fluid distension, synovial proliferation, and neovascularization can completely disappear on follow-up. Conversely, hypoechoic thickening of the bursal wall likely represents an irreversible finding. Small submillimetric variations in bursal wall thickness on follow-up mostly correspond to interpersonal or intrapersonal variations and are probably best considered of no clinical significance. The efficacy of treatment should be assessed on clinical grounds and not based on the magnitude of imaging findings.
4.2. Subcoracoid Bursa
The subcoracoid bursa is located anterior to the subscapularis muscle and inferior to the coracoid process. Its function is to reduce friction between the coracobrachialis, subscapularis, and short head of the biceps tendons, thus facilitating internal and external rotation of the shoulder.48 The subcoracoid bursa does not communicate with the glenohumeral joint under normal circumstances, but may communicate with the SSB. The percentage of such communication is estimated at 10-55%49-52 and the higher percentage suggest that the communication may be a tiny obliterated remnant that becomes patent when either bursa contains excess fluid.50
4.2.1. Sonographic Technique
The subcoracoid bursa is not visible at US in healthy individuals. However, its expected location should always be scanned, obtaining sagittal images just inferior to the coracoid process. Because of its deep location, sonographic evaluation may require a lower-frequency curvilinear array probe for adequate visualization.
4.2.2. Spectrum of Findings
Sonographic detection of the subcoracoid bursa is always abnormal (figure 4-24) and more conspicuous during external rotation of the shoulder (video 4-8). Subcoracoid bursa should not be confused with the superior subscapularis recess, which may extend anterior to the subscapularis tendon and mimic subcoracoid bursitis. The distinction is important because fluid detected in the superior subscapularis recess at US represent joint effusion (see figure 5-22), whereas fluid in the subcoracoid bursa indicates bursitis.
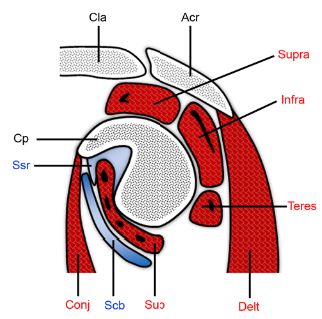
Figure 4-24. Subcoracoid bursa. Schematic drawing of an oblique sagittal view through the glenoid. The superior subscapularis recess (ssr) extends below the coracoid process (cp) and above the subscapularis (sub). The subcoracoid bursa (scb) intervenes between the subscapularis and the conjoined tendon of the short head of the biceps and the coracobrachialis (conj). Acr= acromion. Cla= clavicle. Delt= deltoid. Supra= supraspinatus. Infra= infraspinatus. Teres= teres minor.
The subcoracoid bursa is considered an extension of the SSB when communication between these two structures does occur, sharing common clinical presentation, pathogenesis, and management (figure 4-25). Because of this potential communication, fluid in the subcoracoid bursa should always raise suspicion for a rotator cuff abnormality. Isolated subcoracoid bursitis is rare but has been implicated as a source of shoulder pain. Because of its rarity, the literature describing isolated subcoracoid bursitis is confined to sporadic case reports, with no specific studies on pathogenesis.
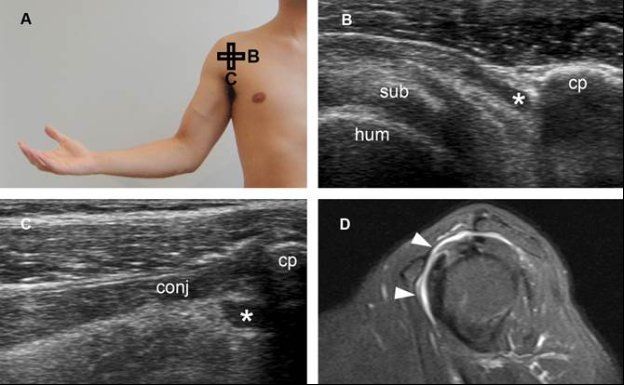
Figure 4-25. Subcoracoid bursitis. [A] Positioning of the probe. [B] Corresponding axial 12-5 MHz US image shows fluid distension of the subcoracoid bursa (asterisk). [C] Corresponding sagittal 12-5 MHz US image shows fluid distension of the subcoracoid bursa (asterisk). [D] Corresponding sagittal STIR MRI confirms subcoracoid bursitis communicating with SSB (arrowheads). Sub= subscapularis. Hum= humerus. Cp= coracoid process. Conj= tendon of the short head of the biceps and the coracobrachialis.
4.2.3. Grading of Subcoracoid Bursitis
As in the case of SSB, we generally do not report measurements of the subcoracoid bursitis. We, however, consider important to inform when a communication between the SSB and subcoracoid bursa is found at US.
4.2.4. Follow-up of Subcoracoid Bursitis
Studies on the follow-up of subcoracoid bursitis are lacking. In the absence of specific data, it is reasonable to consider subcoracoid bursitis as a synonym of subacromial-subdeltoid bursitis for sonographic follow-up purposes.
4.3. Supra-Acromial Bursa
The supra-acromial bursa (SAB) is located on the superior aspect of the acromion and normally does not communicate with the glenohumeral joint.53
4.3.1. Sonographic Technique
The SAB is not visible at US in healthy individuals. Yet, its expected location is usually included in the field of view while performing standard routine scanning of the acromioclavicular joint.
4.3.2. Spectrum of Findings
Supra-acromial bursitis has not been receiving much attention from imaging literature and remains described mainly as case reports of presumptive diagnosis with no histopathological correlation.54,55 On daily basis, it is typically depicted by target evaluation because of point tenderness over the acromion (figure 4-26). Its pathogenesis is unclear. Since the bursa is supra-acromial, not supraclavicular, fluid-filled masses located over the acromioclavicular joint or distal clavicle do not correspond to supra-acromial bursitis. The main differential diagnosis of supra-acromial bursitis is acromioclavicular joint ganglion (see section 1.2.2 in chapter 5).
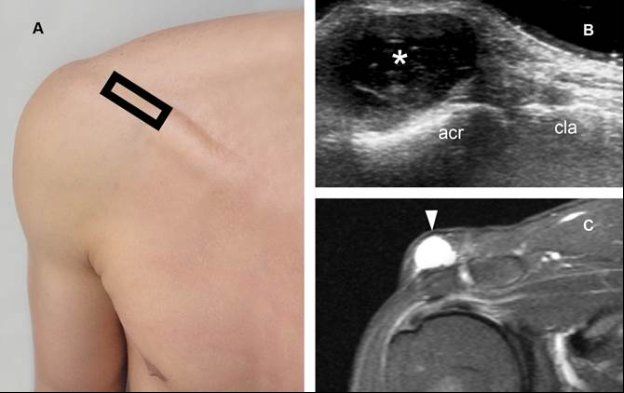
Figure 4-26. Supra-acromial bursitis. [A] Positioning of the probe. [B] Corresponding 12-5 MHz US image shows a fluid-filled lesion (asterisk) located superficial to the acromion (acr). This finding is consistent with the diagnosis of supra-acromial bursitis. Note that the lesion is located superficial to the acromion, not clavicle (cla) nor acromioclavicular joint. [C] Corresponding coronal oblique STIR MRI confirms sonographic finding (arrowhead).
4.3.3. Grading of Supra-Acromial Bursitis
We generally use the ellipse formula to calculate and report the volume of pathological SAB because of its easy access. Also, this information can be useful when considering needle aspiration, though it has no proven influence on prognosis and may not correlate with the intensity of pain.
4.3.4. Follow-up of Supra-Acromial Bursitis
We consider supra-acromial bursitis as a synonym of subacromial-subdeltoid bursitis for follow-up purposes.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree