SPECT-CT and PET-CT of Tumors with an Unknown Primary
Gerhard Goerres
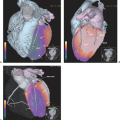
In patients with a metastasis from an unknown primary, the diagnostic workup is directed according to the site where the metastasis was found at presentation. Positron emission tomography (PET) and PET–computed tomography (CT) using fluorodeoxyglucose (FDG) can be used to detect an unknown primary, but to date PET has mainly been evaluated in the setting of patients with squamous cell carcinoma involving neck nodes. It is not well defined when FDG-PET should be used during the clinical workup of a patient: at the beginning or when all other clinical and imaging evaluations failed to identify the primary. Therefore, PET cannot yet be recommended as a routine procedure in all patients with a metastasis from an unknown primary. However, in patients with a metastasis in a neck lymph node, whole-body PET can identify the primary not only in the neck area but also at distant sites in the thorax or abdomen, which often is the primary site of an adenocarcinoma (see Fig. 36.3).
Clinical Background
A metastatic cancer of an unknown primary has been defined as a biopsy-confirmed malignancy for which the site of origin is not identified by routine clinical workup (1). Metastatic cancer of an unknown primary has also been referred to as MUO (metastasis of unknown origin), CUO (cancer of unknown origin), and CUP (cancer of unknown primary) syndrome. Patients with metastatic carcinoma from an unknown primary site represent up to 15% of all patients with cancer who present to medical centers and up to 5% of patients with solid tumors referred to the oncologist (2). The mean survival of patients with an unknown primary has been reported to be less than 6 months (3).
The diagnostic workup has to characterize the biology of the tumor and the site of origin as well as the extent of tumor involvement. The approach used to identify the primary site varies depending on the site of metastasis found at presentation. A workup includes a complete history, a physical examination, routine laboratory tests, and imaging. Most important is the careful pathologic examination of the specimen, including specialized studies, such as immunoperoxidase staining, evaluation of the receptor status, and even electron microscopy. Because the diagnostic workup is directed according to the site of the metastasis found at presentation, if liver metastases are found, for example, further evaluation will center on the search for gastrointestinal tumors. In this case, the workup will include a digital rectal examination with a test for occult blood in the stool, as well as endoscopic evaluation, ultrasonography, or computed tomography (CT), because the patient may suffer from colorectal cancer. In addition, pelvic and breast examinations in women and prostate and testicular examinations in men should always be performed. If, for example, the neck is the site of metastasis, clinical and endoscopic examination of the mouth, nose, paranasal sinuses, pharynx, larynx, and esophagus should be carried out.
In some patients who present with a metastasis, the primary tumor is not unknown, since it is definitively
characterized as a known type of tumor on histopathological grounds. However, the primary site of such an occult tumor is not always detectable. This situation can arise, for example, in women with an axillary metastasis of a breast cancer (4,5) or in patients with melanoma or liver metastases of a neuroendocrine gastrointestinal cancer (6). Another possibility is that the lesion suggested to be a metastasis is in fact the primary site. For example, breast cancer can arise in ectopically localized breast tissue in the axilla, mimicking metastasis. Furthermore, it has been postulated that the primary site may disappear due to an immunologic response of the body, which can occur in patients with melanoma and in patients with a squamous cell cancer of the neck presenting with a lymph node metastasis.
characterized as a known type of tumor on histopathological grounds. However, the primary site of such an occult tumor is not always detectable. This situation can arise, for example, in women with an axillary metastasis of a breast cancer (4,5) or in patients with melanoma or liver metastases of a neuroendocrine gastrointestinal cancer (6). Another possibility is that the lesion suggested to be a metastasis is in fact the primary site. For example, breast cancer can arise in ectopically localized breast tissue in the axilla, mimicking metastasis. Furthermore, it has been postulated that the primary site may disappear due to an immunologic response of the body, which can occur in patients with melanoma and in patients with a squamous cell cancer of the neck presenting with a lymph node metastasis.
Imaging plays a major role in identifying individuals who are treatable. The search is directed primarily by the site and the histological diagnosis of the metastasis (7). Usually, morphological imaging tests are performed as first-line examinations. Imaging evaluations of the neck, chest, and abdomen using CT, ultrasound, and magnetic resonance imaging (MRI) are used to identify the primary lesion. A chest x-ray is often the first imaging test and can identify a bronchogenic carcinoma as the primary tumor in a patient who presents with a brain metastasis but who has no clinical symptoms pointing to the lung in the first place. Up to 70% of patients presenting with brain metastases from an unknown primary have bronchogenic carcinoma (8). In women with axillary metastasis, mammography with ultrasound and if necessary MR mammography should be performed. In many centers, patients with an unknown primary will undergo CT scanning from the head to the pelvic floor as a first-line “screening” test.
The Role of Conventional Nuclear Medicine
Although most radiological methods provide structural information on only parts of the body, the molecular imaging tests of nuclear medicine are an ideal adjunct for screening the whole body, which can be relevant for characterizing an unknown primary. For example, whole-body imaging with bone scintigraphy may help to characterize a primary, because a typical pattern of distribution of skeletal involvement, although not specific, may narrow the diagnosis. It has been shown that bone scanning can identify the presence of occult malignancy in patients with musculoskeletal pain, since metastatic cancer is one of the main causes of skeletal pain (9,10). Whole-body imaging with nuclear medicine is often able to identify more lesions than primarily suggested, thus influencing further workup and eventual therapy. Additionally, other nuclear medicine methods, such as gallium 67 scintigraphy, were used to evaluate patients with cancer of unknown origin (11) but have been largely replaced by fluorodeoxyglucose (FDG) positron emission tomography (PET).
Nuclear medicine can provide highly specific information on selected tumors. For example, in a recent study, somatostatin receptor scintigraphy was able to identify the primary site of an unknown neuroendocrine neoplasm in patients with negative results after clinical, radiological, and endoscopic workup (12). In this study, the results of somatostatin receptor scintigraphy lead to relevant changes of patient management and prompted surgical intervention in 17% of the patients (12). The use of single-photon emission computed tomography (SPECT)-CT seems advantageous here, in analogy with the experience with other tumors and PET-CT (see Chapter 43). In patients with suspected recurrent colorectal carcinoma based on tumor marker levels and no clinical and radiological signs of local recurrence or metastases, radioimmunoscintigraphy with monoclonal antibodies has been successfully used. However, to date only a few studies are available that have reported on the use of scintigraphy with radiolabeled antibodies in patients with an unknown or occult primary carcinoma (13). As the sensitivity of radioimmunoscintigraphy is notoriously low and the procedure relatively costly, this method has never gained widespread acceptance.
The Role of PET and PET-CT
Many reports suggest that FDG-PET can aid in identifying the primary site in patients with metastasis from an unknown primary cancer. The use of PET in this clinical situation is best documented in patients presenting with a metastasis in the neck (14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30). The primary tumor is most often located in the upper aerodigestive tract. In about one third of the cases with squamous cell carcinoma, the primary is located in the tonsils, but it may also be found in areas that are difficult to inspect clinically, such as the pyriform sinus and the nasopharynx (28,31). Furthermore, the base of the tongue is an area where the identification of small primary tumors can be difficult, since in the lymphatic tissue of the Waldeyer ring there is physiologic low to intermediate FDG uptake (see Figs. 33.13 and 33.21). Because it is possible to miss small primaries with imaging, some authors suggest that tonsillectomy or adenoidectomy is performed routinely in these patients. Others perform panendoscopy with routine biopsies of the base of the tongue, pyriform sinus, and tonsils. Small primaries in areas with physiologically increased FDG uptake, such as the Waldeyer ring, can be missed with PET. PET, like other imaging tests, is not a good tool for detecting small primaries and micrometastases, and therefore primaries with a size of less than 5 mm have rarely been reported in the literature (24). The variability of FDG uptake in the tonsils, adenoid, and salivary glands and also in muscles of the head and neck can
render image analysis difficult and may result in false-positive and false-negative findings.
render image analysis difficult and may result in false-positive and false-negative findings.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree