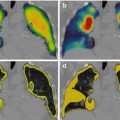
Fig. 2.1
Illustration of the therapeutic windows (TW) concept applied to 90Y-DOTATOC PRRT. The disease control probability (DCP) and kidney NTCP were computed for their respective minimum, median and maximum dosimetry measured by 86Y-DOTATOC PET in the phase 1 clinical study using amino acid infusion [1, 9]. The curves were computed for a disease owning five tumours, and the tissue radiation tolerance parameters were extracted from [6, 10]. For a patient owning both the median tumour and median kidney dosimetry, W3 is a good TW choice giving a probability of 90 % to be curative and of 10 % to get a late renal failure. If his tumour dosimetry is the maximal one observed, then W2 is a better choice avoiding any risk of late renal failure. A patient with the maximal kidney dosimetry observed can be cured only if he also owns the maximal tumour dosimetry observed (W1)
During the last decades, the efficacy of EBRT and the sophistication of the devices used have increased together. From these points of view, the recent CyberKnife system is really impressive [11]. In reality, there is no real innovation regarding the CyberKnife hardware (the knife part): it is the combination of a linear accelerator and of a standard industrial 6-axis robot used in car manufacturing, both existing for three decades. So why this system appeared only recently? The major benefit of the CyberKnife is to allow decreasing the absorbed dose to the critical tissues by increasing the number of different beam paths crossing the patient body to target the tumour. This (the cyber part) required motion tracking and an accurate individual treatment planning that uses state-of-the-art multimodality imaging [12–14] including elaborated Monte Carlo simulations of the absorbed dose spreading along the beam paths. This feasibility results from the continuous development of such treatment planning assessment in EBRT during the last decades [15].
Internal radiotherapy had the good fortune to begin with two pathologies owning a large therapeutic window: the radio-synovectomy and the thyroid cancer 131I radio-ablation. These two therapies were used with success by simply injecting a standard activity. Sometimes, an early success durably formats the behaviours, and despite a lot of efforts spent during two decades, no such ideal radio-compound was found for the other cancers. For the patient’s benefit and also for the long-term future of nuclear medicine, we have to push the available internal radiotherapies to their optimal efficiency by performing an individual treatment planning at the same quality level as that routinely performed in EBRT. Let it be emphasised that a dosimetry method displaying a good dose–toxicity correlation on a patient’s sample is not sufficient: As the goal is to inject to the patient the maximal activity that he can safely receive, the dosimetry has to be accurate on a patient per patient basis.
2.2 SPECT Versus Planar
There are four effects which definitely disqualify the use of planar-based dosimetry in most of internal radiotherapies: (1) γ-rays attenuation-scatter, (2) tissues overlapping, (3) multi-compartment organ and (4) heterogeneous organ uptake.
1.
There is no way to accurately correct gamma rays attenuation in planar acquisition: use of conjugated planar views, even jointly with a planar transmission scan, is a crude approximation. This method is only valid for an infinitely thin organ without any other activity overlapping. Use of a point scatter kernel to correct for the organ’s cross-contamination is hampered by the lack of information about the activity depth distribution. This cross-contamination is very cumbersome regarding that biological half-life of the organs is different and that the critical organs can be located close to tissue owning higher activity, such as liver, spleen, tumour and bowels close to the kidneys in PRRT.
2.
The critical organs can partially or fully be overlapped by higher taking up tissues. Often, this problem is casually considered, and several papers proposed patient dosimetry assessment based on planar views using correction method for the overlapping tissues. But to our knowledge only one [16] presented a validation on phantoms, which should be done for all proposed methods. However, these phantoms were simple: no full overlap, identical effective half-life for the different tissues, and no appearing and disappearing activities (bowel in PRRT). Let it be emphasised that such overlap correction methods have to accurately work for the worst patient case to whom it is not ethically defendable to tell that we cannot do an accurate treatment planning, because we chose to not use the best tool.
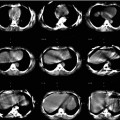
Sandström et al. [17] compared the absorbed dose assessed from conjugate planar views and SPECT/CT in 24 patients imaged 1, 24, 96 and 168 h post-177Lu-DOTATATE therapy. Both modalities were corrected for attenuation: a 57Co transmission scan was performed with the planar modality for this purpose. The planar view to SPECT total kidney absorbed dose ratio ranged from 0.8 to 5.4: 6 patients out of 24 had a relative deviation higher than 40 %. This clearly disqualifies planar imaging in PRRT pre-therapy planning. Garkavij et al. [18] observed the same problem, in 16 patients also treated with 177Lu-DOTATATE, although with a lower maximal planar view to SPECT total kidney absorbed dose ratio: 1.8. The reason explaining this huge discrepancy between planar and SPECT-based dosimetry, in both studies, originated from significant radioactivity overlap as illustrated in Fig. 2.2. In an older 90Y-DOTATOC study, Valkema et al. reported that organs overlapping preventing accurate planar dosimetry assessment occurred in 6 out of 43 patients [19].
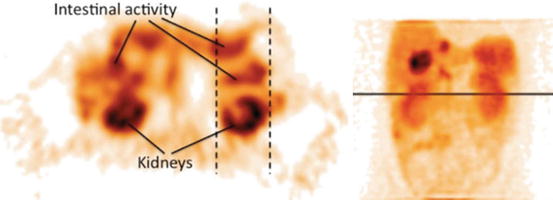

Fig. 2.2
Two images illustrating an imaging situation that results in an overestimated absorbed dose to the kidneys. The activity uptake in the contents of the intestine that overlaps the kidney in the planar image (as indicated by dashed lines) contributes to the absorbed dose, which is not the case in the single-photon emission computed tomography image. Note that the overlapping activity is difficult to detect in the planar images (Reprinted from Garkavij et al. [18] with permission of John Wiley and Sons)
3.
Some critical organs own several compartments displaying different uptakes, biological washouts and radiosensitivities. For example, in PRRT, the renal cortex and medulla represent about 70 and 30 % of the kidney activity, respectively [20]. The critical tissue, i.e. the glomerular, is located into the cortex. The medulla to cortex S-factor is about one fourth of that from the cortex to the cortex [21]. The volume, uptake and biological washout of these compartments are also patient dependent [1, 22]. These three points require separately assessing the number of decays occurring in the medulla and in the renal cortex, which cannot be done in planar view.
4.
Even if the organ has a homogeneous radiosensitivity, i.e. the spatial variations of the radiosensitivity are smaller than the ionising particle range, such as the liver in 90Y-radioembolisation, assessing the intra-organ absorbed dose distribution is still needed. Indeed, studies have shown that the NTCP does not depend only on the mean organ absorbed dose but also on its distribution [23, 24]. NTCP can be calculated using the equivalent uniform dose (EUD) formalism that accounts for the absorbed dose distribution [10]. For homogeneous density organs, the EUD can be computed based on a fast convolution of the SPECT image by a dose deposition kernel, preferably deconvolved by the SPECT system spatial resolution [25].
Lastly, the argument, which is still sometimes advanced nowadays [16], that planar views have to be used because SPECT is too much time consuming is not relevant. Irradiating a patient from the inside is a medical act as serious as irradiating him from the outside and should be done in the same sophisticated way.
2.3 SPECT/CT Versus SPECT
Hybrid SPECT/CT system allows a better co-registration accuracy of the two modalities than that obtain by trying to acquire the patient with exactly the same geometry in two different systems or than to use delicate nonrigid fusion. This favourably impacts the activity quantification. Regarding dosimetry assessment, this also helps to link the activity observed to the tissue owning it.
Activity quantification requires the knowledge of the patient attenuation map. SPECT systems equipped with a gamma ray transmission source are rare. The attenuation map can be derived from the CT Hounsfield values using appropriate rescaling [26]. The issue regarding the additional irradiation received by the patient from the CT performed at the different SPECT time points needed to assess the pharmacokinetics is purely philosophical regarding the four higher order of magnitude of the absorbed dose received during the therapy.
Recent literature review showed that SPECT/CT performed better in absolute quantification than conventional SPECT system [27]. On acquisitions of a torso phantom, Shcherbinin et al. [28] reported errors between 3 and 5 % for the isotopes 99mTc, 123I, 131I and 111In. The phantom contained two sources centrally and peripherally placed with no surrounding activity. For 99mTc in a cardiac torso phantom, Vandervoort et al. [29] reported an error of 8 % in simulation and within 4 % for the acquisition. Both studies included attenuation, scatter and collimator PSF in the iterative reconstruction.
Willowson et al. [30] evaluated SPECT/CT quantification for 99mTc in phantoms and in patients. The acquisitions were first corrected for scattering using a transmission-dependent scatter correction (TDSC) method developed on site and afterwards reconstructed with a commercial OSEM software. The scatter-corrected data, the associated reconstructed data and the co-registered attenuation map were then passed to an iterative Chang attenuation correction algorithm using the CT-derived attenuation correction map. Last, a dead-time correction was performed. In the torso phantom, the relative deviation of the total liver-specific activity assessment was 2 %. Clinical evaluation in 12 lung ventilation/perfusion studies after injection of calibrated 99mTc-MAA activity gave a relative deviation ranging from −7.4 to 3.7 % (mean absolute relative deviation of 2.6 %).
Beauregard et al. [31] evaluated a commercially available SPECT/CT system in quantitative 177Lu imaging using the manufacturer’s iterative reconstruction algorithm that included CT-based attenuation correction, scatter correction using a triple-energy acquisition window and collimator PSF. In addition, they added a camera sensitivity calibration function of the count rate in order to correct for the dead time. They performed seven acquisitions of a 20 cm diameter phantom with background activity ranging from 0 to 1 GBq including two cylindrical active sources independently ranging from 0 to 0.7 GBq. The deviation activity ranged from −9.5 to 4.1 % and from −14.9 to 4.3 %, for the whole phantom and for the sources, respectively. The total body activity deviation on five treated patients versus the injected activity (mean ± std activity = 8.9 ± 0.9 GBq) ranged from −4.9 to 0.0 % with dead-time correction (from −15.2 to −10.2 % without).
Those studies show that SPECT/CT can be used for individualised dosimetry treatment planning. The maximal safe activity found to be injected can be reduce by 10 % in order to account for the current quantification accuracy.
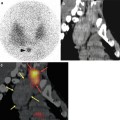
Ahmadzadehfar et al. [32] evaluated the impact of 99mTc-MAA SPECT/CT on SIRT treatment planning and its added value to angiography in 90 studies performed on 76 patients. The accurate co-registration of the two modalities obtained with a hybrid SPECT/CT system allows determining in a robust way which tissue corresponds to the activity observed (Fig. 2.3). Extrahepatic accumulation was detected by planar imaging, SPECT and SPECT/CT in 12, 17 and 42 % of examinations, respectively. The sensitivity for detecting extrahepatic shunting with planar imaging, SPECT and SPECT/CT was 32, 41 and 100 %, respectively, all with a specificity higher than 93 %. They concluded that 99mTc-MAA SPECT/CT is valuable for identifying extrahepatic visceral sites at risk for postradioembolisation complications.
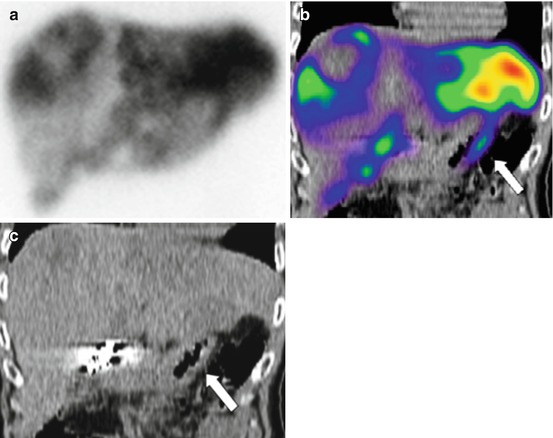

Fig. 2.3
Duodenal accumulation (arrows) in a patient with colorectal cancer, not definable on planar images: planar scan (a), SPECT/CT coronal view (b) and CT coronal view (c) (Reprinted from Ahmadzadehfar et al. [32] with permission of the Society of Nuclear Medicine)
2.4 Choice of a Surrogate
90Y is the major used radionuclide in internal radiotherapy which does not own any isotope having an appropriated half-life and emitting γ-rays that can be imaged by SPECT. The choice of the good SPECT surrogate is a crucial point which has not yet been sufficiently investigated. 90Y is mainly used in PRRT, radio-immunotherapy and liver radioembolisation.
When introducing PRRT the community thought that tissues uptake should mainly depend on the peptide, perhaps a little bit on the chelator and marginally on the radionuclide which is confined in the chelator cage. Later, Reubi et al. showed that receptor affinity for a same chelator-peptide also strongly depends on the labelled radionuclide [33], e.g. by a factor 2 when replacing Y by Ga in DOTATATE labelling. However, contrary to tumours, organ uptakes are not always only receptor dependent. For example, an important part of the 90Y-DOTATOC kidney uptake is not receptor dependent and 111In-DOTATOC-based dosimetry has shown a good correlation with kidney toxicity post-therapy [6].
However, this shows that the choice of a radionuclide surrogate for individualised treatment planning in PRRT requires an initial validation proving identical pharmacokinetics along a patient per patient basis. Such studies in PRRT are still lacking. This validation can be performed by imaging the 90Y therapy by bremsstrahlung SPECT or by PET imaging of the compound labelled with 86Y [34], however, both modalities requiring state-of-the-art correction methods.
In radio-immunotherapy, Minarik et al. [35] compared in three patients the absorbed doses obtained from a pre-therapeutic 300 MBq 111In-ibritumomab SPECT/CT to those obtained post-90Y-ibritumomab therapy by bremsstrahlung SPECT/CT using corrections developed on site (see Bremsstrahlung SPECT/CT chapter). The absolute relative differences between absorbed dose computed from 111In and 90Y SPECT/CT were 8.8 ± 13.7 and 8.9 ± 4.0 (mean ± std in %), for the liver and kidneys, respectively. This supports considering 111In as a surrogate of 90Y in radio-immunotherapy. Compared to peptides, the active site in antibodies is located farther from the radionuclide which likely reduces its impact. However, a validation on a larger patient series is still needed.
For 90Y-loaded glass microspheres, Chiesa et al. [24] performed in 35 patients a co-registering of the 99mTc-MAA SPECT with the 90Y bremsstrahlung SPECT. In the 29 patients treated with the same intentional catheter positioning that in the pre-therapeutic study, the biodistribution was markedly different between the two modalities in two patients (7 %) and seems only attributable to the different physical properties of microspheres and MAA.
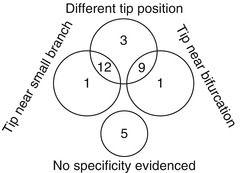
For 90Y resin microspheres, Jiang et al. [36] conducted an interesting study in 81 paired 99mTc-MAA and 90Y bremsstrahlung SPECT performed in 75 patients, the catheter being intended to be set in the same position in the two radioembolisations using angiography. They observed a segmental perfusion difference (SPD) between the two SPECT modalities in 31 patients. Analysing the position of the catheter tip on the two angiograms performed, they noted that 24 SPDs correspond to a different tip position between the two radioembolisations; 2 SPDs occurred with the same tip position but close to an arterial bifurcation or close to a small branch (Fig. 2.4). However, in 5 SPDs no particular specificity was evidenced besides the physical properties of the particles.