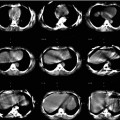
Fig. 10.1
Planar anterior lymphoscintigraphy (a) showing drainage from the injection site in the right breast to the axilla and the internal mammary chain. Multiplanar reconstruction (MRP) with display of similar planes for axial fused SPECT/CT (b) and CT (c) images enables identification of the axillary sentinel nodes (circle). On images generated with maximum intensity projection (MIP) (d) and volume rendering (e), the sentinel nodes are displayed in an anatomical 3D landscape
Fused SPECT/CT images may also be displayed using maximum intensity projection (MIP). MIP is a specific type of rendering in which the brightest voxels are projected into a three-dimensional image allowing improved anatomical SN localisation and recognition by the surgeon (Fig. 10.1d). One limitation of MIP is that the presence of other high-attenuation voxels on CT may complicate the recognition of the vasculature and other anatomical structures. Another limitation of MIP is that it concerns a two-dimensional representation, which cannot accurately depict the actual relationships of the vessels and other structures [4].
Another possibility to support SN localisation is a three-dimensional display using volume rendering. In this modality different colours are assigned to anatomical structures such as vessels, muscle, bone and skin (Fig. 10.1e). This results in better anatomical reference points and incorporates an additional dimension in the recognition of SNs, for instance, in relation to the vasculature. By incorporating a colour display, volume rendering improves visualisation of complex anatomy and 3D relationships.
10.4 Comprehensive Interpretation of Lymphoscintigraphy and SPECT/CT
SPECT/CT does not replace lymphoscintigraphy. It should be considered as a complementary modality. In principle, SPECT/CT aims at anatomically localising SNs already visualised on planar images. However, SPECT/CT can detect additional SNs principally in areas with a high number of lymph nodes such as the neck or with complex anatomy such as the pelvis and abdomen. SPECT/CT is also useful to detect SNs in the vicinity of the injection site. To understand the combined use of lymphoscintigraphy and SPECT/CT, it is necessary to elucidate some aspects related to image interpretation [5]. First of all by acquiring early and delayed planar images, lymphoscintigraphy can identify SNs in a majority of cases. In current protocols SPECT/CT is performed following delayed planar images (mostly 2–4 h after tracer administration). This sequential acquisition helps to clarify the role of both modalities. However, it is necessary to specify some criteria for sentinel node identification on preoperative images. Based on lymphoscintigraphy, the main criteria to identify lymph nodes as sentinel nodes are the visualisation of lymphatic ducts, the time of appearance, the lymph node basin and the intensity of lymph node uptake [6]. Following these criteria visualised radioactive lymph nodes may be classified as follows:
1.
Definitely SNs. This category includes all lymph nodes draining from the site of the primary tumour through its own lymphatic vessel (Fig. 10.2a) or a single radioactive lymph node in a lymph node basin [7].
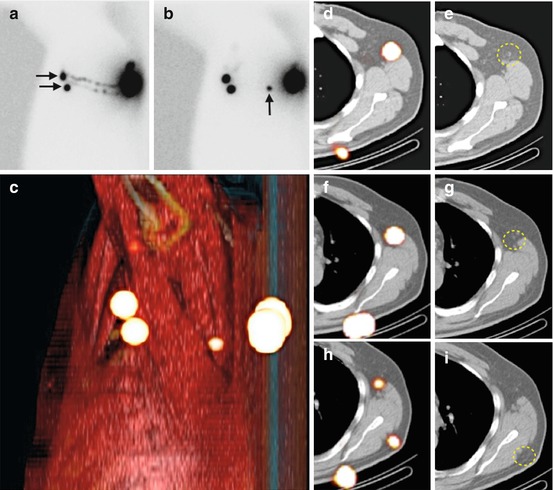

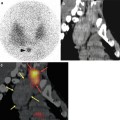
Fig. 10.2
Lateral early planar lymphoscintigraphy (a) in a patient with a melanoma of the back showing lymphatic ducts from the injection site to two lymph nodes (horizontal arrows) in the left axilla. These nodes are considered as definitely sentinel nodes. On delayed lateral planar image (b), an additional node (vertical arrow) is seen in the vicinity of the injection site; this node is highly probable a sentinel node too. Sentinel nodes are anatomically localised using volume rendering (c) and SPECT/CT (d, f, h). On CT (e, g, i) not enlarged sentinel nodes (circles) are seen
2.
Highly probable SNs. This category includes lymph nodes appearing between the injection site and a first draining node (Fig. 10.2b) or nodes with increasing uptake appearing in other lymph node stations.
3.
Less probable SNs. All higher echelon nodes (in trunk and extremities) or lower echelon nodes (head and neck) may be included in this category.
Early planar images are essential to identify first draining lymph nodes as SNs by visualisation of their lymphatic ducts. These nodes (category 1) can be distinguished from secondary lymph nodes (category 3) mostly appearing on delayed planar images.
In other cases a single lymph node is seen on early and/or delayed images. This node is also considered as a definite SN (category 1). However, in some cases SPECT/CT can detect additional lymph nodes in other basins. This may occur, for instance, in the pelvis when two basins are located at the same level and there is superposition of the radioactive nodes on planar images (Fig. 10.3). These nodes can be considered as definite (category 1) or highly probable sentinel nodes (category 2). Less frequently a radioactive lymph node may appear between the injection site and a first draining node; its increasing uptake can confirm this node as a highly probable SN (category 2) and it helps to differentiate this node from prolonged valve activity in a lymphatic duct, which mostly decreases in intensity on delayed images.
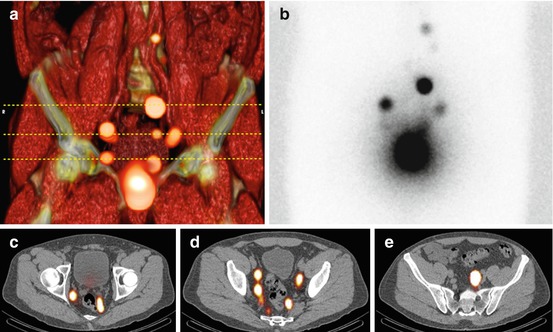

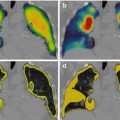
Fig. 10.3
Volume rendering SPECT/CT (a) obtained 2 h after tracer injection into the prostate showing drainage to lymph nodes along the iliac vessels. Horizontal stripped lines indicate from inferior to superior the levels of display of the axial fused SPECT/CT images (c–e). Note that at these levels, SPECT/CT is able to display more sentinel nodes than planar images (b)
10.5 Clinical Relevance of SPECT/CT
10.5.1 Cutaneous Melanoma
In melanoma, SPECT/CT demonstrated to have additional value in 30 out of 85 patients (35 %) by detecting extra SNs not visualised on planar images, or by localising SNs in other basins, or by modifying the incision planning. In 7 patients 12 additional SNs were detected, in two of them the SN contained metastases [8]. In 18 melanoma patients, not only SPECT/CT detected more SNs than planar lymphoscintigraphy (100 % visualisation rate vs. 89 %, respectively) but its additional anatomical information also led to an adjustment of the surgical approach in 4 patients (22 %) [9]. In another study including 35 melanoma patients, SPECT/CT detected additional SNs in 7 of them (20 %) and contributed to modify the surgical approach in 10 (29 %) [10]. Recently, the additional value of SPECT/CT in melanoma has been confirmed in a study comparing the results of 149 patients for whom a SPECT/CT was performed with a group of 254 patients without SPECT/CT. SPECT/CT detected more SNs (average 2.4 against 1.87 without SPECT/CT) and more metastatic SNs (average 0.34 against 0.21). The use of SPECT/CT was associated with a significant higher rate of the 4-year disease-free interval (93.9 %) when compared with the group of patients without SPECT/CT (79.2 %) [11].
10.5.2 Breast Cancer
In a study involving 134 patients, SPECT/CT demonstrated additional value in 48 of them (42 %) by detecting extra SNs or by adjustment of the incision planning. In 15 patients 19 additional SNs were visualised by SPECT/CT not just in the axilla but also in the interpectoral and intramammary. Four of these SNs contained metastases. The SPECT/CT information led to a more precise or to an extra incision [12]. In another study including 41 patients, SPECT/CT led to accurate anatomical SN localisation in the axilla differentiating between levels I, II and III [13]. SPECT/CT is also useful to identify SNs in patients with overweight. In a study including 122 patients, SPECT/CT was performed in 49 of them who had no SN visualisation on planar images. SPECT/CT led to the detection of SN in 29 of these obese patients (59 %) [14]. In another study including 157 patients, SPECT/CT reached a 95 % visualisation rate and important complementary information was gained based on the detection of SNs in patients with negative planar findings, detection of additional drainage basins and identification of false-positive findings on planar images [15]. Also the evaluation of the low-dose CT component of SPECT/CT was helpful to detect lymph node abnormalities in the draining basin; a node was considered positive if one out of four criteria for abnormality were seen: node size >10 mm in axial transverse diameter, irregular borders, nodes round in shape and inhomogeneous structure. When applying these criteria, the low-dose CT yielded a sensitivity of 36 % in 51 patients, with a positive predictive value of 50 % and a diagnostic accuracy of 69 % for axillary staging [16]. In the same study, the number of SNs visualised by SPECT/CT and low-dose CT was discrepant in 13 patients with more lymph nodes seen on CT.
10.5.3 Head and Neck Malignancies
The lymphatic system of the head and neck includes approximately 250–300 lymph nodes divided into various nodal groups. Further, there are marked variations in the lymphatic drainage depending on the primary lesion site. For instance, scalp melanomas of the frontal zone can drain to different lymph nodes when compared with melanomas of the parietal or occipital areas. Also, for the face and the forehead, drainage to different basins may occur. In the oral cavity, malignancies of the lingual apex may drain to other groups in comparison with well-lateralised lesions in the tongue or floor of the mouth. Due to this high lymph node density and variability in drainage patterns, SPECT/CT appears to be essential not only to accurately identify SNs in an anatomical landscape but also to detect additional SNs in the vicinity of the primary lesions or in patients with aberrant drainage to different lymph node basins. In a study including 58 patients with oral/oropharyngeal carcinoma, 11 additional SNs were detected; one of these nodes contained metastases [17]. In another series including 34 patients, additional SNs were found by SPECT/CT in 15, and in 7 patients the anatomical level of SN location was reclassified [18]. SPECT/CT detected additional SNs in 6 out of 38 (16 %) patients with head and neck melanoma and led to an adjustment of the surgical approach in 11 patients [19]. In another study SPECT/CT modified the surgical approach (more superficial incision or incision at other site) in 9 out of 34 (27 %) patients with head and neck malignancies [20].
10.5.4 Urogenital Malignancies
The lymphatic system of the pelvis is the expected area of drainage of various male and female urogenital malignancies. There are different nodal groups receiving lymphatic drainage from the pelvic structures: the external iliac nodes which medial subgroup includes the obturator nodes, the internal iliac lymph nodes and the nodes in the trajectory of the common iliac vessels. SNs from malignancies of the prostate, bladder, cervix and endometrium have been found in these iliac basins. However, aberrant drainage to the para-aortic and interaortocaval nodes as well as to SNs in the proximity of the anterior abdominal wall is also possible. SPECT/CT is particularly useful to anatomically localise SNs in the pelvis and abdomen. In prostate cancer, SN visualisation rate increased from 91 % for planar scintigraphy to 98 % when SPECT/CT was performed. SPECT/CT also depicted more SNs than planar images (average 4.3 vs. 2.2 SNs) in 46 patients. Forty-four percent of the SNs containing metastases were only visualised by SPECT/CT [21]. In 37 out of 121 prostate cancer patients (31 %), SPECT/CT localised SNs outside the area of extended pelvic lymph node dissection which is often used to stage the pelvis; these SNs were found in different locations: presacral, Cloquet’s node, inguinal, para-aortic, abdominal wall, pararectal, behind the common iliac artery and lateral to the external iliac artery [22]. In treated prostate cancer patients, SPECT/CT revealed a higher percentage of SNs (80 % vs. 34 %) outside the pelvic para-iliac region in comparison to untreated patients [23]. In another study SPECT/CT findings led to a 25 % impact of lymphatic drainage on conformal pelvic irradiation in 23 prostate cancer patients [24].
In testicular cancer drainage to nodes along the abdominal aorta and cava is usually found, but in some cases SNs are also found in the trajectory of the funicular vessels. SPECT/CT identified interaortocaval or paracaval SNs in 5 patients with right-sided testicular cancer and para-aortic SNs in 5 patients with left-sided tumours; in three patients SNs along the funicular vessels were detected [25].
In renal cell carcinoma lymphatic drainage was seen in 14/20 patients (70 %) and SPECT/CT detected 26 SNs. Most frequent SNs were located para-aortically but also drainage to retrocaval, hilar, celiac trunk and thoracic (internal mammary chain, mediastinal and pleural) SNs was seen [26].
In a series of 41 patients with cervical cancer, SPECT/CT did increase the SN detection rate to 95 % and was useful to depict unilateral drainage in 19 patients [27]. In another study concerning 44 patients with high-risk endometrial cancer, SPECT/CT identified SNs in 34 of them (77 %) with a total of 110 depicted SNs. The most frequent location was the external iliac chain (71 %) but drainage to para-aortic SNs was also usual (44 %). SPECT/CT was able to localise the only pelvic metastatic lymph node that was not depicted on planar images [28].
10.5.5 Other Malignancies
Preoperative SPECT/CT for SN localisation in gastrointestinal malignancies has not systematically been used. In stage Ia non-small lung cancer, SPECT/CT visualised SNs in 39/63 patients (62 %) and was useful to anatomically depict SNs in hilar and mediastinal lymph node basins [29].
10.6 General Indications of SPECT/CT
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree