Vascular anomalies encompass a collection of diagnoses that differ greatly in terms of clinical presentation, natural history, imaging findings, and management. The purpose of this article is to review diagnostic imaging findings of vascular malformations and vascular tumors, excluding the central nervous system, that occur beyond childhood. A widely accepted classification system created by the International Society for the Study of Vascular Anomalies provides a framework for this review, focusing on the entities most likely to be encountered by general radiologists, although several rare but clinically important entities are also reviewed.
Key points
- •
Clinical history and physical examination findings are often helpful when interpreting imaging of vascular anomalies in both pediatric and adult patients.
- •
The International Society for the Study of Vascular Anomalies classification provides a framework for diagnosis and management of vascular anomalies.
- •
Imaging features of vascular anomalies carry considerable overlap, and biopsy may be required in cases that remain indeterminate after diagnostic imaging.
Introduction
Vascular anomalies encompass a wide range of diagnoses that differ in terms of clinical presentation, natural history, imaging findings, and management. Vascular anomalies are typically diagnosed in the first 2 decades of life. Clinical history and physical examination alone may allow for correct diagnosis in some cases, but diagnostic imaging is often necessary to make an accurate imaging diagnosis. Radiologists can help establish a diagnosis in clinically indeterminate cases, as well as recommend additional imaging in cases of suspected syndromic association or multiorgan involvement. Evaluation by a multidisciplinary vascular anomaly clinic can provide valuable insight into the diagnosis and management of vascular anomalies.
Mulliken and Glowacki devised a cell-oriented classification of vascular anomalies in 1982, delineating entities based on pathophysiology, namely endothelial cell characteristics. Vascular tumors, such as hemangiomas, being true neoplasms, exhibit endothelial proliferation and hyperplasia, whereas vascular malformations do not. Vascular malformations are categorized by predominant channel type, including capillary, venous, lymphatic, arterial, and combinations thereof. This classification was adopted by the International Society for the Study of Vascular Anomalies (ISSVA) in 1996 and last revised in 2018.
ISSVA is a multispecialty group that created the most widely accepted classification system for vascular anomalies. The ISSVA classification provides a standardized nomenclature that takes into account imaging findings, clinical presentation, lesion histology, natural history, and management. Radiologists should use accurate ISSVA terminology when evaluating vascular anomalies, because the use of improper or nonuniform terminology may lead to diagnostic confusion and preclude appropriate clinical management.
The overarching goal of this article is to provide an imaging-focused overview of noncentral nervous system (CNS) vascular anomalies beyond childhood to heighten the understanding of the often challenging diagnosis of vascular anomalies particularly for general practicing radiologists. Vascular anomalies seen in childhood only, including infantile hemangioma, are therefore not discussed in this review.
Imaging techniques
There is considerable overlap of imaging features of various vascular malformations, vascular tumors, and other diagnoses. Doppler ultrasound (US) and magnetic resonance (MR) imaging are the current primary diagnostic imaging modalities used to evaluate vascular anomalies, which are frequently complimentary in establishing a diagnosis and treatment plan. Invasive imaging, including arteriography and venography, is beyond the scope of this article, and is not discussed.
Radiography and Computed Tomography
Radiography and computed tomography (CT) provide limited value in evaluation of vascular anomalies due to poor lesion conspicuity relative to US and MR imaging, as well as exposure of the patient to ionizing radiation. Recent publications have proposed use of multiphasic 4D computed tomography angiography (CTA) performed at 70 kVp with dual source CT as a low-radiation alternative to contrast-enhanced MR imaging, although this technique is likely best suited to patients unable to undergo MR imaging.
Ultrasound
US is often the first-line imaging modality for suspected vascular anomalies in both pediatric and adult patients. Grayscale US allows for evaluation of lesion architecture and Doppler imaging with spectral tracings allows for characterization of lesion flow dynamics. US is widely available, inexpensive, does not use ionizing radiation, does not require sedation, and allows for real-time evaluation with dynamic maneuvers. Limitations of US include operator dependency, a smaller field-of-view, difficulty evaluating deeper and extensive lesions, and inability to penetrate bone and air. US can also be used to monitor response to therapy, when applicable.
MR Imaging
MR imaging plays a key role in the diagnosis of vascular anomalies via multiplanar imaging with superior soft tissue discrimination. Noncontrast MR imaging delineates lesion characteristics, cellularity, extent, and relationship to surrounding structures, such as neurovascular bundles. Contrast-enhanced MR imaging, including time-resolved MR angiography, allows for assessment of lesion dynamics and enhancement patterns. Similar to US, MR imaging can be used to monitor response to therapy when US is not a reasonable alternative.
Optimal MR imaging protocols vary by institution. Vascular anomaly MR imaging evaluation should include multiplanar T1-weighted sequences, with and without fat suppression, for an anatomic overview, fat-suppressed T2-weighted sequences or short tau inversion recovery sequences for lesion extent, and early and delayed contrast-enhanced T1-weighted fat-suppressed sequences for enhancement characteristics. Optional noncontrast sequences, such as T2 gradient recalled echo (GRE) may help identify the presence of hemosiderin, calcification, and high-flow vessels, and diffusion-weighted imaging allows for evaluation of lesion cellularity. MR angiography often proves extremely helpful, particularly dynamic time-resolved MR angiography, as this sequence demonstrates lesion blood flow patterns previously only possible via conventional angiography.
Spectrum of imaging manifestations of vascular malformations and tumors beyond childhood
Vascular Malformations
Vascular malformations arise via errors in vascular morphogenesis, and lack the increased mitotic activity and cellular proliferation that characterizes vascular tumors. Vascular malformations are always present at birth; however, they may be inconspicuous at that time. Vascular malformations grow commensurate with the patient until puberty, and never involute. Vascular malformations can be considered as “high flow” or “low flow” according to the presence or absence of an arterial component, respectively, and this distinction often guides therapeutic decisions. The low-flow category includes capillary, venous, and lymphatic malformation, whereas the high-flow category includes arteriovenous malformation and arteriovenous fistulas.
Low-Flow Vascular Malformations
Capillary malformation
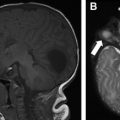
Capillary malformation (CM) presents as a red or pink discoloration of the skin ( Fig. 1 ), and is primarily a clinical diagnosis. Diagnostic imaging of isolated CM is usually not required, although may be performed to evaluate for the presence of a soft tissue component, limb hypertrophy, or a concomitant high-flow component. US and MR imaging may show thickening of the skin and subcutaneous plane, with hyperemia manifested as increased color Doppler flow or contrast enhancement, respectively.

CMs may be seen as part of multiple syndromes, including Sturge-Weber, Klippel-Trénaunay, and Parkes Weber, among others. Therefore, it is important to be familiar with entities associated with CMs to guide imaging investigations in possible syndromic patients.
Venous Malformation
Common venous malformation
Venous malformations (VMs) are the most common vascular anomaly, with an estimated prevalence of 1%; however, this prevalence is likely underestimated due to misdiagnosis and variability of terminology used in the literature. , VMs comprise abnormal tortuous and dilated veins lined by a thin endothelium. VMs are found in the head and neck in 40% of patients, the extremities in 40%, and the trunk in 20%, although they may also be present in the viscera. ,
As with all vascular malformations, VMs are present at birth; however, because they grow commensurate to the patient, they may escape detection until later in life. Likewise, VMs in deep locations, including intramuscular and visceral, may not become symptomatic until the hormonal influence of puberty. VMs are expected to have reached full size in the adult patient, although increases in size can be seen in the setting of hormonal influences, such as menstrual cycling, pregnancy, and with spontaneous intralesional thrombosis.
Morphologic imaging patterns of VMs may include focal dilation of a vein, multiple dilated tortuous dysplastic veins, and multiple mass-like cavitary or spongiform venous channels. Various imaging classification systems for VMs have been proposed, although they are not commonly used in diagnostic practice, as management options are similar throughout.
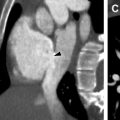
Radiography and CT have limited value in the diagnosis of VMs, with the exception of phleboliths, which are considered nearly pathognomonic ( Fig. 2 ). Bony distortion, including organized periosteal reaction or hypoplasia, may occur due to the presence of an adjacent VM, although this finding is nonspecific.

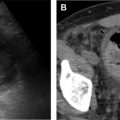
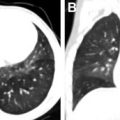
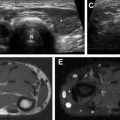
US is often used as first-line imaging in cases of suspected VMs. Up to 98% of VMs appear heterogeneous on grayscale US, with 82% appearing hypoechoic relative to adjacent structures, 10% hyperechoic, and 8% isoechoic. Evidence of a solid component on US should suggest a diagnosis other than VMs, although care must be taken to differentiate intralesional thrombosis from a solid component. Phleboliths, although nearly pathognomonic, are seen in only 16% of cases on US. Dynamic maneuvers, such as application of a tourniquet, can be performed during an US examination and may provide additional information. A superficial VM is expected to be compressible during US examination, although it may be noncompressible in the presence of an intralesional thrombus ( Fig. 3 ). VMs may show engorgement dependent on positioning, Valsalva, or placement of a tourniquet ( Fig. 4 ).


Doppler US of VMs is expected to demonstrate internal flow in 84% of patients, monophasic in 78%, with absence of Doppler flow being the second most common appearance. , Fluid-fluid levels may be present in VMs due to its low-flow nature, although they are not specific for this diagnosis. The presence of arterial flow can be seen due to adjacent and crossing arteries, as well as in cases of complex malformation with a capillary component, although a true arterial component should not be present with VMs.
VMs on MR imaging may be trans-spatial, and can involve subcutaneous and deep tissues, muscle, bone, solid organs, and joint spaces. VMs are commonly iso- to hypointense on T1-weighted imaging and hyperintense of T2-weighted imaging. Fat-suppressed T2-weighted imaging most accurately depicts lesion extent ( Fig. 5 ). Hyperintense T1-weighted foci can be seen in the presence of intralesional thrombus, whereas T1-weighted and T2-weighted hypointense foci may correspond to thrombi, phleboliths, and areas of previous treatment or vascular septa ( Fig. 6 ). Hypointense foci within a VM on spin echo imaging may mimic flow voids, although GRE imaging can help to differentiate as flow voids appear hyperintense. The presence of intralesional flow voids would indicate high-flow vessels, and should therefore suggest an alternate diagnosis.


Postcontrast imaging of VMs demonstrates variable enhancement depending on timing of image acquisition, ranging from early homogenous enhancement to delayed heterogenous enhancement. , Dynamic time-resolved MR angiography is valuable to establish lesion flow characteristics. Perilesional edema or presence of an enhancing soft tissue component should prompt consideration of biopsy to evaluate for malignancy. Posttreatment change may manifest as regional T1-weighted and T2-weighted hypointense signals, with corresponding lack of enhancement ( Fig. 7 ).

Venous malformation associated with blue rubber bleb nevus syndrome
Blue rubber bleb nevus syndrome (BRBNS) is a rare, sporadic entity comprising multifocal VMs, involving the skin in all patients, and commonly the gastrointestinal (GI) tract. , Cutaneous VMs in BRBNS are typically small, on the order of 1 to 3 cm diameter, and may be painful. VMs in BRBNS may be located anywhere along the gastrointestinal tract, most commonly in the small bowel. GI VMs may manifest as unexplained anemia or GI bleeding, although they may also be the lead point for an intussusception. ,
VMs in BRBNS demonstrate the same imaging features expected in common VMs. Nuclear medicine-tagged red blood cell scan has been described as the most sensitive imaging modality for detection of GI lesions, and may demonstrate focal or multifocal avid radiotracer uptake. Fluoroscopic upper and lower GI examinations may demonstrate multiple polypoid filling defects anywhere along the GI tract, mimicking a polyposis syndrome. CT and MR imaging may not identify GI tract lesions, and therefore may be most helpful to evaluate for the presence of cutaneous and solid organ involvement. ,
Glomuvenous malformation
Glomuvenous malformation (GVM) is a developmental hamartoma of glomus body origin, previously referred to as glomangioma, and is categorized as a VM subtype by the 2018 ISSVA classification. , GVMs may be sporadic or demonstrate autosomal dominant inheritance.
GVMs present as clustered blue or purple tender vascular nodules in the cutaneous and subcutaneous plane. Lesions may be solitary, or multifocal and plaque-like, demonstrating a “pebbly” or cobblestone appearance, which helps to differentiate this entity from common VMs. ,
US of GVMs may reveal decreased compressibility compared with common VMs, and phleboliths are not expected. , MR imaging demonstrates a T1-weighted hypointense to isointense, T2-weighted hyperintense cutaneous or subcutaneous multilobulated, septated lesion with low-signal thin septa ( Fig. 8 ). Dynamic time-resolved MR angiography demonstrates patchy arterial phase enhancement. Early venous shunting may be present, although lack of dilated feeding arteries or draining veins help to distinguish this entity from arteriovenous malformation (AVM).

Lymphatic Malformation
Common lymphatic malformation
Lymphatic malformations (LMs) are benign vascular malformations arising from abnormal development of the lymphatic system, and are the second most common type of vascular malformation after VMs. LMs may be microcystic, macrocystic, or mixed, according to the size of the cysts, with microcystic lesions containing cysts smaller than 1 cm.
LMs can occur anywhere throughout the body, most commonly in the head and neck, with approximately 50% occurring in this area. LMs commonly involve the subcutaneous tissues, and are often trans-spatial, involving multiple additional tissue planes. Sudden increased size of LMs in adult patients can be seen in the setting of bleeding secondary to trauma or infection, hence the commonly seen physical examination findings of bluish discoloration of the skin or erythema, respectively.
Radiography has a limited role in the evaluation of LMs, although it may demonstrate foci lucent areas with bone involvement. CT of macrocystic LMs may demonstrate a circumscribed, multiseptated lesion with large locules and internal simple fluid density, although fluid density may be increased in the setting of hemorrhage or proteinaceous fluid. CT of microcystic LMs demonstrates nonspecific ill-defined fat stranding and infiltration, with no visible cystic spaces or discrete mass, often involving the subcutaneous plane.
Grayscale US of macrocystic LMs demonstrates multiple anechoic cystic spaces separated by thin septa ( Fig. 9 ). Grayscale US of microcystic LMs demonstrates an ill-defined hyperechoic area with or without visible tiny cystic spaces, with the hyperechoic appearance owing to the numerous wall interfaces within the lesion ( Fig. 10 ). The presence of millimetric cutaneous or subcutaneous fluid-filled spaces within the area of concern during an US examination suggests a diagnosis of LM. Doppler US may show septal flow, although arterial or venous flow should not be present within the cystic spaces. Peripheral hyperemia can be seen in the presence of infection or inflammation of the LM, although it should otherwise be absent. Fluid-fluid levels may be present within the cystic spaces, although this feature is not specific for LM, and is also seen in VM. Dynamic maneuvers during an US examination can help to differentiate LMs from VMs; in distinction from VMs, valsalva should not lead to engorgement of an LM, and compression should only deform, not collapse, the LM cystic spaces. The presence of millimetric cutaneous fluid-filled vesicles within the area of concern at the time of a US examination suggests a diagnosis of LM.