Clinical Presentation
The patient is a 60-year-old woman complaining of headache, imbalance, lightheadedness, and left-leg numbness. She also complains of increasing numbness in her hands with some hand discoordination. She has had significant neck and bilateral shoulder pain, possibly somewhat worse on the left side. On examination, she demonstrates −1 paresis of the biceps, triceps, wrist extensors, wrist flexors, and interossei musculature. Her grasp is diminished, particularly on the right.
Imaging Presentation
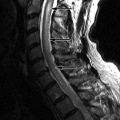
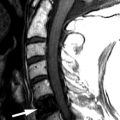
Sagittal T2-weighted and axial fat-saturated, T2-weighted magnetic resonance (MR) images were obtained and demonstrate a long segment region of decreased signal intensity along the posterior aspect of the vertebral bodies from C3-C5, which is greatest at the C3-4 level. The findings represent a disc herniation at C3-4 in a patient with ossification of the posterior longitudinal ligament. There is severe spinal stenosis at C3-4 and a long segment region of abnormal increased T2 signal intensity in the cervical spinal cord from mid C3 to the C5-6 level compatible with spinal cord ischemia and/or myelomalacia ( Fig. 71-1 ) .

Discussion
Cervical spinal stenosis can be defined as any narrowing of the spinal canal that causes compression of the contents of the spinal canal because of a mismatch between the available space in the spinal canal and its contents. If the stenosis becomes severe enough, myelopathic symptoms can arise. Cervical myelopathy is the most serious condition that can arise from cervical spinal stenosis. The signs and symptoms of this condition depend on which part of the cervical cord is compressed.
Cervical spinal stenosis can be divided into congenital, developmental, and acquired causes. Congenital cervical stenosis often affects the craniovertebral junction. Patients with malformation of the dens or achondroplasia or who have Down syndrome or Klippel-Feil syndrome can present with cervical spinal stenosis. Developmental stenosis is usually caused by short pedicles. As a person ages, degenerative changes ensue. Normally, a person would not be symptomatic from degenerative changes until they were moderate or severe. However, with short pedicles, one tends to see symptoms with much milder degenerative changes because the available spinal canal space is already diminished in anterior-posterior diameter and area. Therefore, patients with developmental stenosis tend to have symptoms at a younger age. Acquired stenosis is much more common than congenital or developmental stenosis. Degenerative stenosis is the most common type of acquired stenosis. Other causes of acquired cervical spine stenosis include ossification of the posterior longitudinal ligament, ossification/thickening of the ligamentum flavum, rheumatoid arthritis with pannus formation, ankylosing spondylitis, Paget’s disease with basilar impression, and metastatic disease.
Disc degeneration and the degenerative cascade that follows is the most common cause of acquired cervical spinal stenosis. Generally, the midcervical region is most affected. In healthy individuals, the cervical intervertebral disc is similar to the lumbar intervertebral disc, consisting of an annulus fibrosis and nucleus pulposus. In the first and second decades of life, lateral tears occur in the annulus fibrosis. These tears, over time, enlarge and extend toward the medial aspect of the disc. As we age, the degenerating disc cannot bear or transfer load because of annular fissuring, disappearance of the nucleus pulposus, and dehydration of the disc. There is increased load upon the uncovertebral joints, which become flattened to accept the additional load. This then puts greater stress on the vertebral endplates. Osteophytes develop because of periosteal irritation at the vertebral margins in order to increase the weight-bearing surface of the endplates, stabilizing the adjacent vertebra. The osteophytes can become quite large, bringing the degenerated disc material along with them. The intervertebral disc may calcify to further stabilize the vertebral motion segment. The ligamentum flavum may hypertrophy and buckle into the spinal canal. The combination of the osteophyte/disc complex and thickened ligamentum flavum causes narrowing of the central spinal canal. The combination of the flattened, degenerated uncovertebral joints and facet joint hypertrophy can cause neural foraminal narrowing. Vertebral subluxations, secondary to facet degeneration and ligamentous laxity, can further contribute to spinal stenosis and foraminal narrowing.
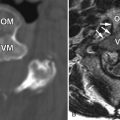
Ossification of the posterior longitudinal ligament (OPLL) is a condition in which there is pathologic ossification of this ligament in the cervical and/or thoracic spine. When this ossification occupies enough of the spinal canal, it will result in cervical spinal stenosis, which can lead to myelopathy and/or radiculopathy secondary to chronic pressure on the spinal cord and nerve roots. The posterior longitudinal ligament (PLL) is a band of collagen and elastin fibers that extends along a line along the posterior margins of the vertebral bodies from the atlas to the sacrum. The PLL is narrower and weaker than the anterior longitudinal ligament (ALL), which extends along the anterior margins of the vertebral bodies, also from the atlas to the sacrum. The fibers of both of these ligaments are firmly attached to the annulus of the intervertebral discs and the corner of the vertebral bodies. The ligament is widest at the disc spaces and narrowest at the mid-vertebral levels. The ligament is also thicker centrally and progressively thins out laterally. OPLL usually occurs in patients over 40 years of age and is very rare until the third decade. OPLL has been well studied in East Asian countries with an incidence of 2% to 4%. The prevalence of OPLL in other countries has not been well studied. OPLL has been estimated to have a prevalence of 0.12% in a radiology review. One quarter of North Americans and Japanese patients with cervical myelopathy exhibit OPLL. Most often, OPLL is found in the upper cervical spine (70%, C2-C4) and less often in the upper thoracic spine (15%, T1-T4). Cervical OPLL occurs twice as often in males as in females.
Ossification of the ligamentum flavum (OLF) is more common in the lumbar and thoracic regions; however, it may occur at the atlantoaxial region as well. OLF is common in the Japanese population, affecting up to 20% of Japanese patients greater than 65 years of age, with rare reports in Caucasians and people of African descent. Neck pain and arm weakness are the most common symptoms; however, with greater spinal stenosis, bowel and bladder dysfunction can occur.
Cervical stenosis can be a dynamic process. Typical computed tomography (CT) and magnetic resonance imaging (MRI) demonstrate static abnormalities that can cause cervical stenosis. However, the size and shape of the available space in the central spinal canal can change with motion, particularly flexion and extension. Chen and colleagues measured these changes in human cadavers and discovered that from flexion to extension, disc bulging decreased the spinal canal diameter by 10.8% and ligamentum flavum bulging decreased the spinal canal diameter by 24.3%. Similar changes were seen with axial loading on the cervical spine.
Upper cervical spinal stenosis, often due to congenital abnormalities, may cause neck pain and restricted movement. With greater stenosis, these patients can suffer from respiratory paralysis or even sudden death. Developmental stenosis secondary to short pedicles can have myelopathic symptoms and/or radicular pain. Acquired degenerative cervical spinal stenosis may have myelopathic symptoms such as weakness in the arms and hands; a staggering, wide gait; and interosseous atrophy. With foraminal and lateral recess narrowing, shoulder and arm pain can appear.
Imaging Features
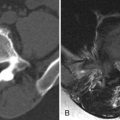
The role of imaging is not only to define whether spinal stenosis is present but also to determine what the cause is and what the relative contribution of bony versus soft tissue spinal stenosis is. Plain radiographs are often not useful. Although radiographs can demonstrate changes of disc space narrowing and osteophytes, the relative contribution of these abnormalities to spinal stenosis is not evident. Myelography has been used in the past but has been supplanted by less invasive cross-sectional imaging. Myelography indirectly demonstrates spinal stenosis as a narrowing of the contrast-filled thecal sac ( Fig. 71-2 ) . One then needs to deduce whether the narrowing is caused by soft tissue and/or a bony substance. Myelography is very insensitive to abnormalities outside of the central canal and does not allow for visualization of abnormalities lateral to the midneural foramen. A combination of myelography followed by CT can improve visualization of both the bony detail and nerve root compression ( Fig. 71-3 ) , but because myelography is invasive and requires an intrathecal injection of contrast material, MRI and plain CT are the imaging modalities of choice for spinal stenosis.


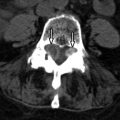
Both CT and MRI can demonstrate the presence of spinal stenosis. CT has a clear advantage in the evaluation of the bony contribution to spinal stenosis (osteophytes, facet degeneration) ( Fig. 71-4 ) , whereas MRI has the advantage of soft tissue contributions to spinal stenosis (disc bulge/herniation, ligamentous hypertrophy, synovial cysts). Whether by CT or MR imaging, the imaging characteristics of spinal stenosis show a change in shape of the spinal canal from its rounded or oval shape to a more irregular or flattened appearance ( Fig. 71-5 ) . There may be displacement or obliteration of the epidural fat adjacent to the thecal sac or in the neural foramen ( Fig. 71-6 ) . With MRI, there may be loss of cerebrospinal fluid (CSF) around the nerve roots on T2-weighted sequences. If stenosis is severe enough, there may be increased T2 signal intensity within the cervical cord compatible with cord ischemia, which could lead to cord infarction ( Fig. 71-7 ) . Foraminal narrowing can be evaluated on the sagittal MR images (which tends to overestimate foraminal narrowing) or from reformatted sagittal images from the CT axial data.