Author
Patients (lesions)
Histology
Total dose (range)/No. of fractions (range)
Spinal cord dose
Follow-up (months)
Local control
Myelopathy
Moss et al. [3]
16 (16)
Hemangioblastoma
21 Gy (20–25 Gy)/2 (1–3)
NR
33.5
92 %
0 %
Selch et al. [4]
9 (20)
Hemangioblastoma
12 Gy/1
D max 12 Gy and V10 = 10 %
51
90 %
0 %
Daly et al. [5]
25 (18)
Hemangioblastoma
20 Gy (18–30)/1
D max 22.7 Gy
33.7
86 %
4 % grade ≥2
18–25 Gy/(2–3)
D max 14.1 Gy
Sinclair et al. [7]
15
AVM, type II
20.5 Gy (15–25)/3 (2–5)
NR
27.9
All patients had radiographic reduction of nidus
0 %
Malignant Tumors
Primary Tumors
Chordomas
Although quite rare, chordomas are the most common primary malignant tumor of the spine. Standard of care for these tumors is en bloc resection with wide margins. In the setting of an incomplete or intralesional resection, which often arises in the presence of epidural disease, adjuvant radiation to a total dose of at least 65 Gy is indicated [8–10]. In a report by Terezakis et al., seven chordomas were treated with postoperative image-guided conventionally fractionated radiation therapy to a median dose of 6,600 cGy (range, 5,396–7,080 cGy) in 180–200 cGy per fraction. The median planning target volume was 164 cm3 (range, 29–1,116 cm3). With a median follow-up of 17.4 months (range, 2.1–47.3 months), one patient developed in-field recurrence. There were no patients who experienced myelopathy.
Proton radiation therapy is an established treatment option for the treatment for sacral chordomas, and outcomes correlate with dose. In a study by Park et al. [11], 27 patients with sacral chordomas (16 primary and 11 recurrent), most of whom underwent surgical resection (78 %), received photon/proton radiation, and were followed for a mean of 8.8 years. The mean dose for primary tumors was 71 Gy (E) in 36 fractions versus 77 Gy (E) in 41 fractions for recurrent tumor. The mean diameter for primary tumors was 8.6 cm (range, 2.8–19.3 cm) versus 5.7 cm (range, 2.7–9 cm) for recurrent tumors. Local control rates for surgery and radiation were 12/14 (86 %) for primary versus 1/7 (14 %) for recurrent chordomas. Among patients receiving definitive radiation, doses of 73 Gy (E) or more resulted in greater rates of local control [11]. The authors recommend 77.4 Gy (E) to areas of gross residual disease or unresected chordomas. Dose escalation resulted in high rates of local control. Proton radiation facilities are not readily accessible; however, this prompted photon dose escalation with modern image-guided techniques, enabling hypofractionation.
In the study by Jiang et al. [12], both intracranial and extracranial chordomas were treated to a dose of 32.5 Gy (range, 18–50 Gy) in 1–5 fractions. The average tumor volume was 16.1 cm3 (range, 2.4–45.9 cm3). With a reasonable follow-up of 34 months (range, 2–131 months), overall tumor control for the whole cohort was achieved in 11 of 20 patients (55 %). Eight of those patients had a reduction in tumor size. Moreover, patients with spinal chordomas (seven in total) had improved symptoms and higher tumor control than intracranial lesions, 71 % versus 46 %. One patient with no evidence of disease radiographically had collapsed at L3 and required surgical decompression.
Henderson et al. reported on a series of 24 lesions, the majority of which (61 %) involved the spine or sacrum. The median tumor volume was 128 mL (range, 12.0–457.3 mL) to a median dose of 35 Gy (range, 24.0–40.0 Gy) in 5 fractions. Median follow-up was 46 months (range, 7–65 months) and actuarial 5-year local control was 59.1 %. Toxicity profiles were similar to those reported in other series, and no patients experienced grade 3 or 4 neurologic complications [13].
The authors currently employ image-guided high-dose radiosurgery neoadjuvantly prior to surgical resection. In cases where gross total resection is achievable, a neoadjuvant approach may facilitate intralesional resection. The neoadjuvant approach was inspired by a case report in which a patient with spinal chordoma underwent definitive single-fraction, high-dose, image-guided radiation to the tumor. Unfortunately, 4 months after treatment the patient underwent L3 corpectomy due to symptoms of mechanical instability [14], which revealed near-complete necrosis of the resected chordoma. Results of our institutional experience with the neoadjuvant approach will be published shortly.
Spinal Metastases
The prevalence of spinal metastases in North America is over 100,000 patients each year. Over 40 % of cancer patients will develop metastatic disease in the spine, the bulk of which is extradural. Highly effective and durable palliation is clinically significant, especially in the context of longer life expectancies secondary to improved systemic agents [15]. Conventionally fractionated radiation therapy has been an integral component of the treatment paradigm for spinal metastases; one or two open fields are used to deliver the radiation dose bound by spinal cord tolerance [16]. The evolution of image-guided technology and breakthroughs in treatment planning has revolutionized the management of spinal metastases [17–19]. In situations where the tumor is in close proximity to the spinal cord, safe dose escalation is possible via steep dose gradients. While these techniques have been used in the primary-irradiation, postoperative setting, as well as re-irradiation, the optimal dose, patient-selection, and delivery method has yet to be defined.
Primary Irradiation: Single Fraction
The ideal fractionation schema for palliation of bone metastases has been studied extensively [14, 20]. One of the most eminent trials, RTOG 9714, randomized patients to 8 Gy × 1 versus 3 Gy × 10. The single-fraction, low-dose arm resulted in higher rates of retreatment with radiation, 18 % versus 9 % [20]. In a contrasting meta-analysis of over 3,260 patients, clinical outcome was unchanged with single-fraction, low-dose (median, 8 Gy) versus fractionated radiation (median, 4 Gy × 5) [14]. Palliation of pain, however, favored single-fraction, low-dose radiation. The recently updated meta-analysis now includes over 5,000 patients and findings show no significant difference in pain relief [21].
Particularly for spinal metastases, long-term outcomes of conventional fractionation or single-fraction, low-dose radiation for the treatment of spinal metastases have been reported in a prospective study by Nagoya University. Approximately 101 patients with spinal tumors received 40 Gy in 20 fractions, were followed for more than 24 months (11 months for dying patients and 53 months for survivors), and reported 67 % pain relief [22]. Similarly, in a randomized study by Maranzano et al., metastatic cord compression received 8 Gy × 2 over one well versus a split course of 5 Gy × 3 plus 3 Gy × 5. At 33 months follow-up, pain relief was similar for short- versus long-course patients, 56 % and 59 %, respectively [23]. In general, the mean pain relief expected from conventional fractionated radiation is 70 % (range, 56–100 %).
Radiographic local control rates are similar to pain relief with conventionally fractionated radiation, about 77 %. One of the most critical prognosticators of recurrence is tumor histology. Favorable or radiosensitive histologies include breast cancer, prostate cancer, lymphomas, seminomas, and myelomas. Unfavorable or radioresistant tumors include sarcomas, melanomas, renal cell carcinomas, gastrointestinal cancers, and non-small cell lung cancer [24]. In the aforementioned study by Maranzano, radiosensitive histologies had significantly improved median survival and duration of neurologic motor improvement with spine radiation, 10 versus 3 months (p = 0.001) and 11 versus 3 months (p = 0.001), respectively [23]. Additionally, for the long-term survivors of the Nagoya study, durable response at 53 months was greater in the radiosensitive tumors (30 % versus 10 %) [22].
Successful outcomes for spinal metastases should be differentiated from osseous metastases and should include close radiographic follow-up. Single-fraction, high-dose image-guided intensity-modulated radiation therapy (IG-IMRT) is proved to be highly effective in both pain and radiographic control, irrespective of tumor histology. Pain improvement is approximately 85 % and often immediate, occurring within days of treatment [19, 24]. In general, pain usually decreases within weeks of treatment. Long-term radiographic tumor-control rates are excellent at 90 % [25–27]. Moreover, radioresistant tumor histologies that respond poorly to conventionally fractionated radiation such as renal cell carcinoma or melanoma are potentially cured locally with single-fraction, high-dose IG-IMRT.
Gerszten et al. reported on a mixed population of 500 patients with spinal metastases. In approximately 65 patients, single-fraction, high-dose IG-IMRT was the primary treatment modality. The mean tumor dose was 20 Gy (range, 12–25 Gy), while mean follow-up was 21 months (range, 3–53 months). Long-term control and pain improvement was excellent at 90 % and 86 %, respectively. There were no cases of myelopathy [28].
In a larger series of 93 patients with 103 spinal metastases treated with radiation as the primary treatment modality, Yamada et al. reported excellent overall radiographic local control of 90 % [29]. In this series, patients received single-fraction, high-dose IG-IMRT of 18–24 Gy (median, 24 Gy). An example of isodose distribution for high-dose IG-IMRT is shown in Fig. 44.1. The median follow-up was 15 months and all patients underwent close surveillance with magnetic resonance imaging at each scheduled follow-up visit to assess local control. The 2-year estimated cumulative incidence of local failure with death as a competing risk was 7.5 %, with median time to failure of 9 months. Tumor histology did not predict for local control on multivariate analysis, while radiation dose was the only significant predictor. The local control rates for low-dose (18–23 Gy, mean 20.8 Gy) versus high-dose (24 Gy) radiation were 80 % and 95 %, respectively (p = 0.03). In all cases, death was due to systemic disease progression rather than local failure. The median time to death after treatment was 10 months (range, 1–39 months), while median overall survival was 15 months. At the time of death, all but one patient had no evidence of local failure.
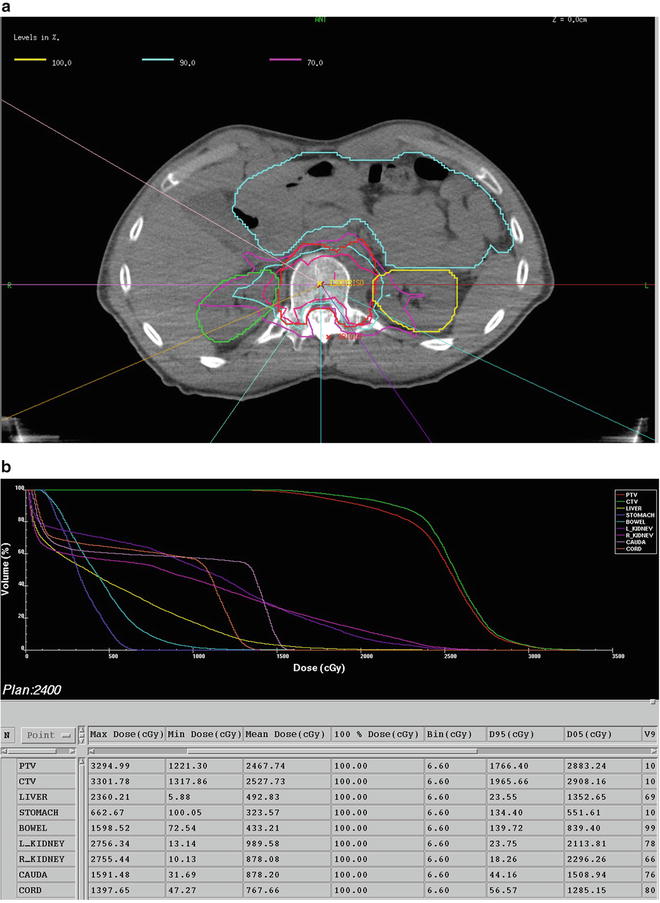

Fig. 44.1
Forty-eight-year-old man with radioresistant liposarcoma and single metastasis at T12 treated with 2,400 cGy in a single fraction. Computed tomography myelogram is used to define the true cord. (a) Isodose distribution revealing a high-dose gradient in the epidural space. (b) Dose volume histogram reflecting the dosimetric advantages of image-guided intensity-modulated radiation therapy and limited dose to the true cord
Due to the observed dose response, the planned dose distributions were retrospectively reviewed [30]. Dosing parameters, including D min and D95, were examined for patients experiencing local failure and compared with the whole cohort. The median D min (10.8 Gy) for patients experiencing local failure was significantly lower than those patients experiencing local control (D min, 15.7 Gy). Moreover, when D min was <15 Gy, local failure rates were 16 %. However, when D min was >15 Gy, no failures were observed in any histology. It would appear that underdosing to even a small subvolume of treatment target is clinically significant.
Adjuvant Postoperative: Single Fraction
If the spinal cord is compromised by tumor, such as with high-grade epidural compression or spinal instability, decompressive surgery is the standard of care, resulting in improved neurologic and functional outcomes [30]. Residual tumor at the dural margin is at risk for recurrence; thus, the majority of metastatic spinal tumors require adjuvant radiation for microscopic or gross disease. High-dose, single-fraction IG-IMRT has demonstrated superior outcomes compared with conventionally fractionated adjuvant radiation for radioresistant histologies. Postsurgical intervention, the tumor environment is hypoxic as well as radioresistant and the 1-year local control rate is dismal at 20–30 % [31]. Additionally, treatment is often delayed, with conventional radiation secondary to wound healing. Conversely, since skin dose is negligible due to steep dose gradients, IG-IMRT may be given early in the postoperative period.
In a small series of 18 patients which included highly radiosensitive histologies such as multiple myeloma, postoperative single-fraction IG-IMRT was delivered with a mean dose of 11.4 Gy (range, 6–16 Gy). The results were outstanding, with 92 % of patients having stable or improved neurologic symptoms at a median follow-up of 7 months [32].
In another series by Moulding et al., 21 patients received adjuvant postoperative high-dose, single-fraction IG-IMRT to a higher median dose of 24 Gy (range, 18–24 Gy) [33]. In this series, the overwhelming majority of patients (95 %) had radioresistant histologies. Patients were grouped into high- and low-dose IG-IMRT groups of 24 Gy and 18 or 21 Gy, respectively. The overall local control for the entire cohort was 81 % at a median follow-up of 310 days. When stratified by dose group, the high-dose IG-IMRT group had a lower 1-year estimated local failure risk than the low-dose IG-IMRT group, 6.3 % versus 20 %. Overall survival was not significantly different among high-dose versus low-dose IG-IMRT.
Adjuvant IG-IMRT after surgical decompression and instrumentation is highly effective in ensuring local control and sustaining neurologic recovery, especially for radioresistant histologies.
Salvage Reirradiation Dose and Hypofractionation
Tumor recurrence or progression after conventional radiotherapy at the original site is common, on the order of 50–80 % at 2 years [22]. Furthermore, recurrences after a previous course of conventional radiation are more likely to be radioresistant since they ultimately failed radiation and thus require robust radiation doses for successful salvage. Reirradiation with conventional means is limited by spinal cord tolerance and the concern for radiation-induced myelopathy. In instances where additional conventional radiation or surgery is not appropriate, IG-IMRT is a successful option.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree