US
CT
MR
Cyst/pseudocyst
Posterior acoustic enhancement may contain echoes
Attenuation similar to water with a thin non-enhancing wall. Attenuation may be increased if infected or complex, and the wall may enhance if infected
Increased T2 signal intensity with variable T1 signal intensity and non-enhancing thin walls when not infected
Gamna-Gandy bodies
Hyperechoic with or without shadowing
Hypointense T2 signal intensity, blooming on T1 in-phase images, and no enhancement
Hemangioma
Solid echogenic or complex cystic appearance, and similar enhancement to background splenic parenchyma
Capillary – Iso- or hypoattenuating on non-contrast imaging, with homogeneous contrast enhancement. Cavernous – solid and cystic components with heterogeneous enhancement of the solid components following contrast administration
Hypo- to isointense to normal spleen parenchyma on T1-weighted images. Hyperintense to spleen on T2-weighted images. Variable enhancement
Hamartoma
Echogenic, occasionally with cystic or calcified components. Early enhancement with rapid washout
Isoattenuating with background spleen and often only appreciated secondary to contour abnormalities. May enhance on postcontrast early arterial phase images
Isointense with background spleen on T1-weighted images, heterogeneous on T2-weighted images, and persistent enhancement on delayed imaging
Lymphangioma
Similar appearance to cysts, except Doppler evaluation shows intrasplenic vascularity coursing about the periphery
Similar appearance to cysts
Multiloculated T2-hyperintense fluid with T2-hypointense septa. Variable T1 signal intensity without enhancement
Littoral cell angioma
Mottled echotexture without discrete lesions or multiple solid lesions of variable echogenicity
Iso- or hypoattenuating lesions become isoattenuated or remain hypoattenuated following contrast administration
Usually hypointense on T1 and T2 images, but can have some increased T2 signal intensity depending on hemosiderin content
Hemangiopericytoma
Hypoechoic with early arterial phase enhancement and decreased enhancement during portal and delayed phase imaging
Multilobular lesions with speckled calcifications as well as enhancing solid nodular components and/or septa
Hyperintense T2 signal intensity with corresponding hypointense T1 signal intensity
Hemangioendothelioma
Hypoechoic masses, with anechoic areas and disorganized color flow in the setting of necrosis and tumoral angiogenesis
Hypoattenuating masses with enhancement of solid components (to a lesser degree than background splenic parenchyma). Necrotic and hemorrhagic areas do not enhance
Heterogeneous T1 and T2 signal intensity with areas of decreased T1 and T2 signal intensity related to hemosiderin
Peliosis
Echogenic spleen with varying hypoechoic foci. Rapid central enhancement of the spleen with associated centripetal enhancement
Multiloculated blood-filled spaces with well-defined septa, occasionally with fluid-fluid levels. Significant enhancement of the lesions or enhancement of the dependent fluid
Increased T2 signal, occasionally hemorrhagic with increased T1 signal,occasionally hypervascular
Inflammatory pseudotumor
Hypoechoic with calcific components
Hypoattenuating mass with calcifications and heterogeneous enhancement
Hyperintense T2 signal intensity (occasionally hypointense), hypo- to isointense on T1-weighted imaging, and heterogeneous enhancement
Lipoma
Echogenic
Fat attenuation without enhancement
Increased T1 and T2 signal, which saturates on fat-suppressed images
Abscesses
Cystic lesions that progressively enlarge and may contain gas, possibly with subcapsular or extracapsular extension
Similar to ultrasound appearance
Hypointense T1 and hyperintense T2 signal intensity with minimal rim enhancement
Sarcoidosis
Variable hypoechoic to slightly hyperechoic inhomogeneous nodules without enhancement
Hypoattenuating nodules that do not enhance
Hypointense T1 and T2 signal without enhancement. Caseating granulomas are T2-hyperintense with peripheral hypointensity
Table 45.2
Imaging characteristics of malignant splenic lesions
US | CT | MR | |
|---|---|---|---|
Angiosarcoma | Complex heterogeneous mass with foci of necrosis and/or hemorrhage. Hypervascularity on color Doppler interrogation in the solid portions | Similar to ultrasound appearance. May be hyperdense, or have punctate or large radial calcifications | Variable T1 and T2 signal related to necrosis, hemorrhage, and hemosiderin. Heterogeneous enhancement |
Littoral cell angiosarcoma | Imaging characteristics of littoral cell angioma, with a more infiltrative or solid appearance | Imaging characteristic of littoral cell angioma, with a more infiltrative or solid appearance | Imaging characteristic of littoral cell angioma, with a more infiltrative or solid appearance |
Pleomorphic undifferentiated sarcoma, fibrosarcoma, and leiomyosarcoma | Rare with no distinguishing characteristics. Can be cystic, solid, or complex masses | Rare with no distinguishing characteristics. Can be cystic, solid, or complex masses | Rare with no distinguishing characteristics. Can be cystic, solid, or complex masses |
Kaposi sarcoma | Splenomegaly with multiple echogenic nodules. Heterogeneous echogenicity of the background parenchyma may be present | Spleen may be homogeneous or show hypoattenuating nodules that are iso- or hypoattenuating on delayed post-contrast images | Hyperintense on T2-weigted images |
Lymphoma | Variable appearance from iso- to hypoechoic. May have increased through transmission. Hypoechoic with peripheral enhancement following contrast administration | Hypo- or isoattenuating without contrast enhancement | Iso- to hypointense on T1- and T2-weighted imaging. Lesions are better appreciated on post-contrast images as they are well circumscribed |
Leukemia | No imaging abnormality. Occasional splenomegaly | No imaging abnormality. Occasional splenomegaly | No imaging abnormality. Occasional splenomegaly |
Cystadenocarcinoma | Unilocular or multilocular large cysts | Unilocular or multilocular large cysts | Unilocular or multilocular large cysts |
Metastases | Most commonly hypoechoic, but can be hyperechoic (e.g., colon). Inhomogeneous when necrosis is present | Decreased attenuation with peripheral enhancement. Calcification can be seen with primary cystadenocarcinomas | T2-hyperintense signal intensity and hypo- to isointense T1 signal intensity with variable enhancement |
45.2 Anatomy
The spleen is a ductless glandular organ that is situated between the gastric fundus and the diaphragm (Fig. 45.1). The organ has diaphragmatic and visceral surfaces. The visceral surface can be further divided into gastric (anterior) and renal (posterior) portions. The gastric surface is in direct contact with the posterior wall of the stomach and tail of the pancreas. The medial aspect of the gastric surface is directed anteromedially and is termed the splenic hilum, where vessels and nerves enter and exit. The renal surface is anatomically associated with the superoanterior surface of the left kidney and sometimes the left adrenal gland. The colic surface sits upon the splenic flexure, phrenicocolic ligament, and usually the tail of the pancreas [2].
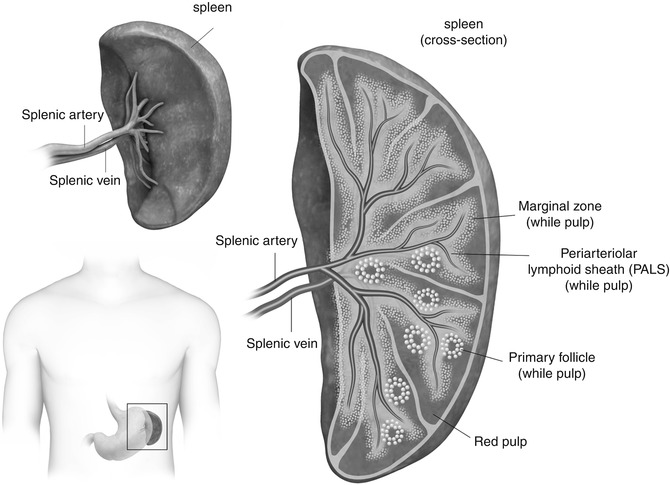

Fig. 45.1
Illustration demonstrates position of the spleen in the left upper quadrant of the abdomen, its gross anatomy including the splenic hilum, vasculature and anterior surfaces, and cross-sectional anatomy showing the components of the spleen including red pulp, white pulp, and marginal zone
The spleen is held in position by two membranes – splenorenal ligament and gastrosplenic ligament [2], both of which are derived from the dorsal mesentery [3]. The splenorenal ligament and extends between the spleen and left kidney, through which the splenic vessels course. The gastrosplenic ligament extends between the spleen and stomach, through which the short gastric and left gastroepiploic branches of the splenic artery course [2].
The spleen functions as a blood-filtering organ that can be divided into three compartments – red pulp, marginal zone, and white pulp (Fig. 45.1). The red pulp is composed of splenic cords and rich plexus of venous sinuses. The splenic cords consist of reticular fibers, reticular cells, and macrophages. Various blood cell types are found in the spaces between the splenic cords. The splenic cords are also associated with lymphocytes and hematopoietic cells. The venous sinuses of the red pulp extend up to the marginal zone, where the systemic circulation is screened for antigens and pathogens. Some consider the marginal zone to be part of the white pulp, which is composed primarily of T and B lymphocytes [4, 5].
45.3 Benign Lesions of the Spleen
45.3.1 Splenic Cysts and Pseudocysts
Cysts of the spleen can be divided into true and false (or pseudo) cysts. True splenic cysts are rare entities [6], with primary cysts making up only 20 % of splenic cysts [7]. Congenital or epithelial splenic cysts account for approximately 75 % of true splenic cysts. Generally speaking, congenital cysts are asymptomatic. However, cysts can enlarge and potentially hemorrhage in the setting of trauma. Additional potential complications include infection and rupture, possibly requiring partial or complete splenectomy [8].
True simple cysts are anechoic with posterior acoustic enhancement on ultrasound. Sometimes thin septa may be present. Internal echoes can be seen in the setting of hemorrhage. Calcifications, if present, are linear reflections with possible associated acoustic shadowing. Infected cysts may show acoustic enhancement of the thin wall and internal echoes (sediment). Ruptured cysts show discontinuity of the thin wall, with anechoic fluid extending beyond the normally spheroid shape of a cyst [8].
On CT, simple cysts have internal attenuation similar to water with a thin, non-enhancing wall. However, the thin wall may enhance if infected. In addition, if the cyst is complex, septations and/or calcifications may be seen. Hemorrhagic and infected cysts with sedimentation show higher attenuation than simple water [8].
On MRI, cysts have increased T2-weighted signal intensity with non-enhancing thin walls, and variable T1-weighted signal intensity, depending on the presence or absence of hemorrhagic products and/or sedimentation. Small foci of calcification are often difficult to identify due to lack of signal but can be seen as blooming on in-phase T1-weighted gradient-recalled images [8].
Pseudocysts make up about 75 % of nonparasitic splenic cysts. The main difference between these secondary cysts and primary cysts is that the wall is composed of fibrous tissue in secondary cysts (not an epithelial lining). Radiographically, these cannot be distinguished from primary cysts. True cysts generally do not need follow-up imaging, but it may be beneficial to follow-up pseudocysts if elicited by trauma to ensure their stability or involution [8].
45.3.2 Gamna-Gandy Bodies
Gamna-Gandy bodies represent hemorrhagic foci within the spleen, which result from portal hypertension [9, 10]. These lesions are composed of fibrous tissue associated with hemosiderin and calcium. Given these characteristics, on ultrasound, Gamna-Gandy bodies appear as hyperechoic foci in the background of normal splenic parenchyma, with or without posterior acoustic shadowing depending on quantity of calcifications. Sclerotic splenic veins associated with portal hypertension are seen as channels of spectral reflectors in the spleen. With MR imaging, the lesions are normally hypointense on T2-weighted imaging, bloom on in-phase T1-weighted gradient-recalled echo images, and do not enhance on post-contrast imaging (Fig. 45.2a–d). These findings in the setting of cirrhosis dismiss miliary tuberculosis, histoplasmosis, and disseminated Pneumocystis carinii infection [10].
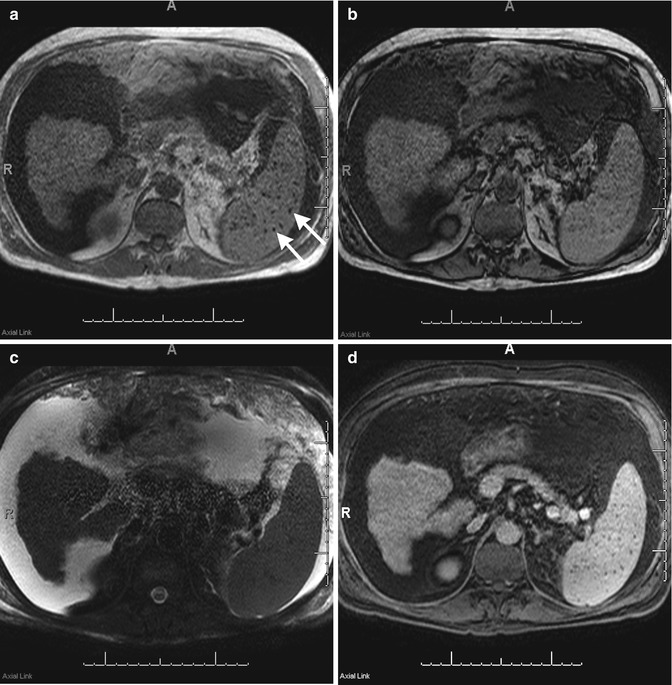

Fig. 45.2
A 59-year-old male with cirrhosis. There is blooming of iron-containing Gamna-Gandy bodies (arrows) on the in-phase T1-weighted images (a) when compared to out-of-phase T1-weighted images (b). In addition the Gamna-Gandy bodies have decreased signal intensity on fat-suppressed T2-weighted imaging (c), and no contrast enhancement on delayed 3D post-contrast fat-suppressed T1-weighted gradient-recalled echo imaging (d)
45.3.3 Hemangiomas
Hemangiomas are the most common primary benign splenic neoplasms. Incidental hemangiomas generally measure <2 cm and commonly occur in the 30–50 years of age. Hemangiomas can be associated with angiomatosis syndromes such as Kasabach-Merritt syndrome, which consists of anemia, thrombocytopenia, and coagulopathy. A complication that can occur, particularly in large hemangiomas, is rupture. Additional complications include hypersplenism and malignant degeneration [1].
On radiographs, a hemangioma can manifest as a left upper quadrant mass or splenomegaly; however, these findings are not specific. Calcifications may or may not be present. On ultrasound, hemangiomas can be well defined or pedunculated and have a solid echogenic or complex cystic appearance. Echogenic foci with acoustic shadowing related to calcifications may be visualized if present [1]. With contrast-enhanced sonography, hemangiomas usually enhance similar to and thus are isoechoic with background splenic parenchyma [11], though this can vary.
On non-contrast CT, capillary hemangiomas are hypoattenuating or isoattenuating lesions, which homogeneously enhance with intravenous contrast [1]. Enhancement of cavernous hemangiomas is associated with only the solid components and not the cystic components [1, 12]. On more delayed imaging, cavernous hemangiomas show discrete heterogeneous, mottled areas of enhancement [12]. Often the cystic components are associated with calcifications. With MR imaging, splenic hemangiomas are hypo- to isointense relative to normal spleen on T1-weighted imaging, whereas they are hyperintense on T2-weighted imaging (Fig. 45.3a, b) [1]. Note that hemangiomas of the liver can show T2-hyperintense signal on diffusion-weighted imaging due to T2 shine-through effect, though there are cases that show restricted diffusion [13] which is also likely the case with splenic hemangiomas. There are three patterns of enhancement associated with hemangiomas: immediate/persistent homogeneous enhancement, early peripheral enhancement with homogeneous delayed enhancement, and peripheral, nodular enhancement with centripetal progression (Fig. 45.3c–f) [14]. If complications occur in large hemangiomas, such as hemorrhage or thrombosis, variable MR characteristics may be seen [1].
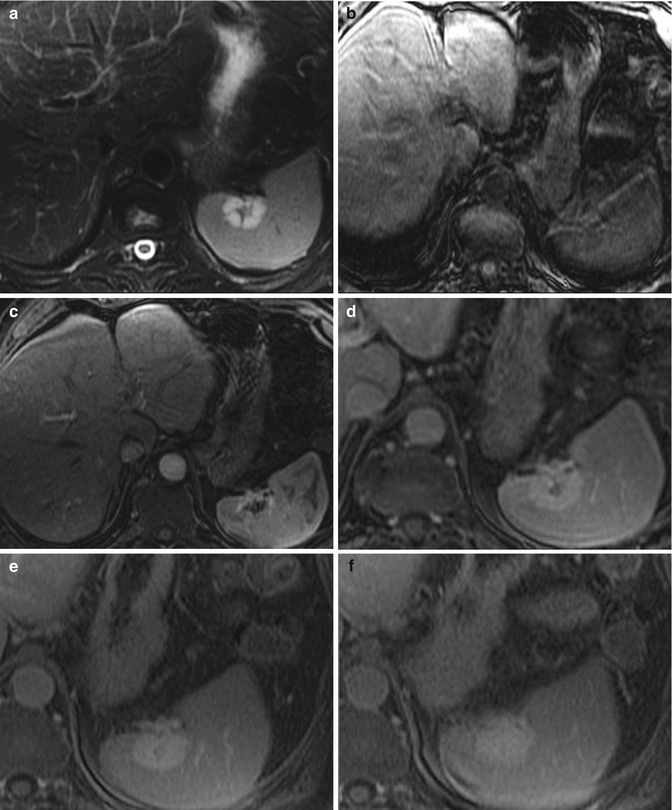

Fig. 45.3
Classic MR imaging characteristics of hemangiomas. Here we demonstrate that the splenic hemangioma is hyperintense on T2-weighted imaging (a) and hypointense on 3D precontrast fat-suppressed T1-weighted gradient-recalled echo imaging (b). During early post-contrast 3D post-contrast fat-suppressed T1-weighted gradient-recalled echo imaging (c), the hemangioma demonstrates discontinuous peripheral nodular enhancement, which fills in centripetally on delayed 3D post-contrast fat-suppressed T1-weighted gradient-recalled echo imaging (d–f)
A case report has shown technetium (Tc-99m)-labeled red blood cell scintigraphy with SPECT can be utilized for confirming presence of splenic hemangiomas [15]. By utilizing early and delayed planar and SPECT images, one can evaluate for focal blood pooling on delayed imaging, as has been shown with hepatic hemangiomas [16]. Alternatively, in Tc99m-labeled sulfur colloid studies, a photopenic defect can occur because the tracer accumulates only in the normal reticuloendothelial system tissue [17].
In general, no intervention or follow-up imaging is needed for hemangiomas, unless the patient is symptomatic. Often patients become symptomatic once hemangiomas have reached a certain size. If the patient has left upper quadrant pain or there is concern for rupture, partial or complete splenectomy may be considered for treatment and postoperative follow-up imaging may be obtained if there is concern for complications.
45.3.4 Hamartoma
Splenic hamartomas are rare benign lesions that can occur at any age, equally in men and women. Similar to hemangiomas, hamartomas are usually found incidentally but may be symptomatic if they are large, presenting as palpable masses, splenomegaly, or ruptured lesions. Hamartomas can be multiple and found in extrasplenic areas and thus are associated with certain syndromes like tuberous sclerosis and Wiskott-Aldrich-like syndrome. Also similar to hemangiomas, thrombocytopenia and anemia may be associated with splenic hamartomas [1].
Hamartomas tend to be solid masses. Thus, on ultrasound, hamartomas appear as echogenic lesions, and sometimes may have cystic or calcific components. These lesions tend to have highly vascular components. In general, on post-contrast sonography, hamartomas demonstrate avid enhancement without early washout [18]. On CT, given the characteristics of the lesion, hamartomas tend to blend in with background splenic tissue, and often times the only defining characteristic is a subtle contour abnormality. In contrast, MR imaging has more defining characteristics. Though hamartomas are T1-isointense with the spleen, they show heterogeneous signal on T2-weighted imaging as well as post-contrast evaluation (Fig. 45.4). On delayed imaging, hamartomas show more uniform enhancement [1].
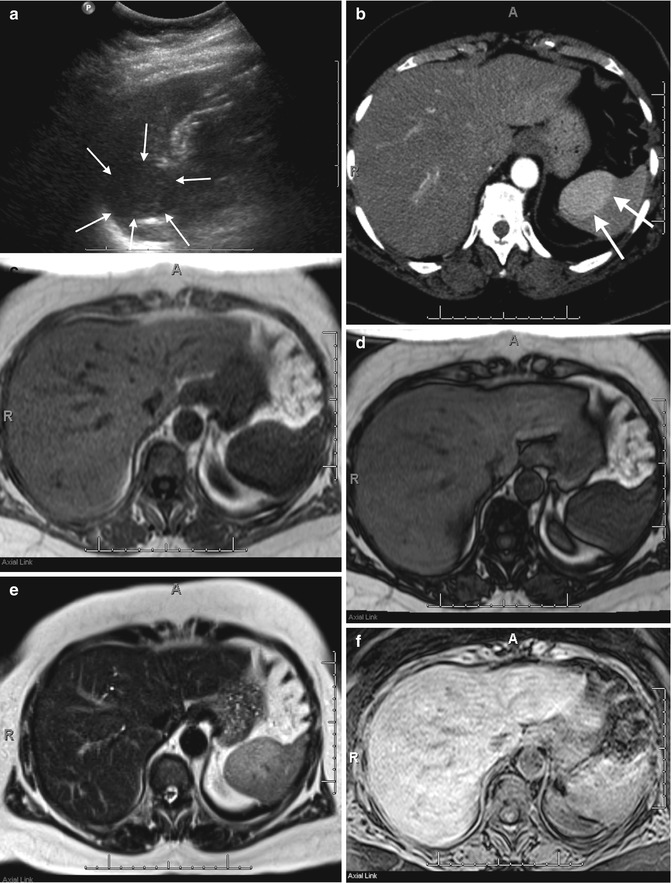
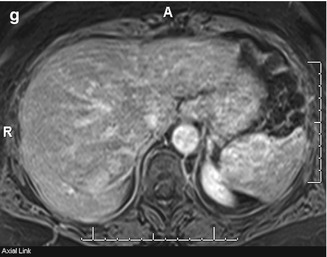


Fig. 45.4
A 71-year-old female with a splenic mass. Sagittal ultrasound of the spleen (a) demonstrates a solid mass arising from the upper pole of the spleen (arrows) with corresponding early arterial phase enhancement (arrows) on an axial-enhanced CT image (b). This mass shows isointense signal with splenic parenchyma on T1-weighted in-phase (c) and out-of-phase (d) imaging but more heterogeneous signal on T2-weighted single shot fast spin echo (e) imaging. The mass shows heterogeneous enhancement when comparing pre- (f) and post (g)-contrast-enhanced fat-suppressed T1-weighted gradient-recalled echo images. These findings are in keeping with a splenic hamartoma, which was pathologically confirmed
With Tc-99m radiolabeled isotopes, “hot spots” are also visualized for hamartomas [19]. It is important to note that given the vascularity of the lesions, they can mimic both benign lesions (such as hemangiomas) and metastatic lesions, and thus are difficult to diagnose with imaging alone.
45.3.5 Lymphangioma
Similar to other benign primary lesions of the spleen, patients with lymphangioma may be asymptomatic or have imaging that shows a large, multicentric mass that requires surgical intervention. Symptoms elicited from this lesion are the result of growth of the lesion that arose during childhood and now has mass effect upon surrounding organs. In addition, if the lesions are very large, bleeding, consumptive coagulopathy, hypersplenism, and portal hypertension may be elicited. When lymphangiomas occur in multiple organs, the process is called lymphangiomatosis [1].
With ultrasound and CT, these splenic lesions are most commonly incidentally detected. These lesions have the appearance of splenic cysts, with a wide range of sizes, and most commonly located in the subcapsular region. Commonly these lesions may have septa, internal debris, and calcifications. Doppler evaluation on ultrasound reveals intrasplenic vasculature coursing along the periphery of the cysts. With contrast-enhanced CT, no significant enhancement is detected [1]. Rarely, FDG uptake with PET/CT may be observed, possibly secondary to lymphatics present within the fibrous septa [20]. With MR imaging, lymphangiomas appear as T2-hyperintense multiloculated lymphatic fluid with intervening T2-hypointense septa. T1-weighted signal is variable in these lesions, depending on the absence or presence of hemorrhagic material and/or other debris (Fig. 45.5). On post-contrast imaging, if malignant degeneration has occurred, it is important to evaluate for soft tissue components [1].
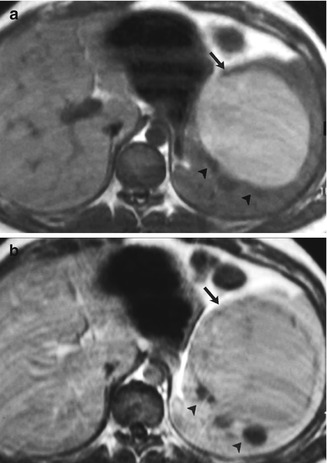

Fig. 45.5
A 27-year-old woman with splenic lymphangioma. A complex cystic mass was detected on a previous routine sonography study and confirmed later on enhanced CT. Axial unenhanced (a) and contrast-enhanced (b) gradient-echo T1-weighted images show a subcapsular multilocular mass with hypo- (arrowheads) and hyperintense (arrow) non-enhancing areas, revealing their cystic nature. Hyperintense areas were secondary to proteinaceous content. Diagnosis was confirmed after splenectomy. Artifact was the result of poor breath holding (With permission from Luna et al. [57])
Majority of lymphangiomas in the spleen are small and do not require surgery. However, larger ones, especially if symptomatic, may require partial or total splenectomy. Follow-up imaging can be considered if there is concern for postsurgical complications.
45.3.6 Littoral Cell Angioma
Littoral cell angioma is a rare vascular splenic lesion that can have benign and/or malignant components. Commonly, these are identified upon work-up of anemia and/or thrombocytopenia in symptomatic patients. Patients also can present with flu-like symptoms and pain. Splenomegaly is almost always associated with littoral cell angioma. The lesion has also been associated with other neoplastic processes including colorectal, renal, pancreatic adenocarcinoma, and meningioma [1].
Littoral cell angiomas usually present as multiple rather than solitary lesions. This pathology has widely variable appearance on imaging. On sonograms, the spleen may present with mottled echotexture without discrete lesions, whereas other cases present as individual lesions with variable echogenicity [1]. Usually with Tc-99m red blood cell scan, littoral cell angioma does not show radiotracer uptake [21]. On CT, these lesions appear as iso- or hypodensities [1, 22, 23] that become isoattenuated [1, 24] or remain hypoattenuated [22] with background spleen on post-contrast imaging. Like on sonography, coalescent lesions can be appreciated. MRI usually shows hemosiderin-filled T1- and T2-weighted hypointense signal given the cellular hematophagocytic capacity. However, some case studies have shown that if the lesions have less hemosiderin content, the lesions may have some T2-hyperintense signal [23, 24].
Given that the symptoms are nonspecific, splenectomy is often performed for definitive diagnosis and treatment.
45.3.7 Hemangiopericytoma
Though hemangiopericytoma is considered a vascular benign lesion, it has high malignant potential. Only 25 % of these lesions arise within the abdomen, and when these do arise within the spleen, the lesions are usually asymptomatic or associated with splenomegaly [1].
These nodular lesions are hypoechoic on ultrasound. With contrast, lesions found in the liver show marked enhancement during arterial sonographic imaging and decreasing enhancement during portal and delayed imaging [25]. This may be similar in the spleen. On CT, the lesions appear as multilobular masses with smaller disseminated lesions throughout the spleen [1]. Speckled calcifications may be associated with the lesions and may show enhancing solid nodular components and/or septa on post-contrast CT images [12]. In some cases, the lesions may not be detected on CT or on Tc-99m sulfur colloid studies. On MR imaging, the lesions show T2-weighted hyperintense signal and T1-weighted hypointense signal [1].
Though these lesions are normally surgically excised, recurrence can occur in as many as 50 % of patients and are aggressive. Since recurrence has been reported even 20 years after initial therapy, close long-term surveillance is necessary [1].
45.3.8 Hemangioendothelioma
Patients with hemangioendotheliomas commonly present with left upper quadrant pain and palpable lesions. Patients also present with hypersplenism, hematologic abnormalities, and metastases. This condition occurs in children and young adult populations [1].
Hemangioendotheliomas are typically hypoechoic masses surrounded by normal splenic parenchyma. Anechoic areas may be present if tumor necrosis is present. On color Doppler images, disordered vascularization may be seen in the presence of tumoral neoangiogenesis. With CT, the lesions appear as hypoattenuated masses with enhancement of solid portions, which may appear hypovascular relative to background spleen. Necrotic and hemorrhagic areas do not show enhancement. These lesions may also have an infiltrative appearance. When the lesion occurs in the spleen, capsular retraction along surface lesions is not observed as is with liver lesions. On MR imaging, hemangioendotheliomas tend to be heterogeneous with hypointense T1- and T2-weighted signal due to presence of hemosiderin [1].