Fig. 13.1
Baseline CT of isolated liver metastases and follow-up imaging at 3 months and 18 months revealing no evidence of progressive disease
In this regard, assessment of tumor response to therapy using functional information could be very helpful in the absence of good anatomical imaging such as 18-fluorodeoxyglucose-positron emission tomography (FDG-PET) scanning modality. PET scans have had a significant impact on the management of patients with many cancers but it has been studied most extensively in NSCLC. Their most important contribution has been in the improvement in the preoperative evaluation of mediastinal lymph nodes over computed tomography (CT) scans alone with increased sensitivity and specificity [32–34] and in staging improving patient selection for curative therapy. FDG-PET has also been prognostic in the pretreatment setting and following induction chemotherapy [35] or definitive chemoradiotherapy to determine tumor response as defined by FDG uptake for predicting outcomes [36, 37]. Following SBRT, PET findings have been mixed with only a few small series reported.
A small prospective series [38] from Fox Chase Cancer Center which included ten medically inoperable patients treated for NSCLC on a phase I dose escalation demonstrated that a drop in the maximum standardized uptake values (SUV) comparing the pre-SBRT and the 3-month post-SBRT PET scan appeared to be a predictor of local control. A larger retrospective experience [39] from the same institution including 26 patients addressed using FDG-PET response (comparing pre-SBRT versus 3-month post-SBRT) as a surrogate of local failure in greater detail. All 26 PET scans were reviewed and interpreted by a single physician who was blinded to the clinical results. FDG-PET scans were analyzed semi-quantitatively by comparing pre- and posttreatment changes in SUV. This study demonstrated two major findings: (1) A lower initial SUV value was the only significant factor associated with an increased risk of local failure (3.4 versus 5.7; p = 0.045) with 4 out of the 5 local failures having a low pre-SBRT SUV (<4). (2) Decreases in post-SBRT SUV of >55 % of its pre-SBRT value significantly decreased the risk of local recurrence. Similar findings were seen when this data was combined with data from the University of Maryland [40] which demonstrated a crude local failure rate of 54.5 % failure rate (6/11) for patients with SUV <4, while only 11 % (4/36) of patients with SUV ≥4 failed locally. Hoopes et al. [41] have described with nearly 50 % of patients at 1 year having SUVmax changes >3.5 from their prospective study and 15 % in their retrospective experience (4 of 28 with a max SUV >2.5). Presumably, though, all were lower than pretreatment. These patients were too compromised to undergo further treatment and were thus followed. They remained without recurrence between 8 and 22 months post these PET scans. This illustrates that the use of a specific SUV value is not useful and therefore trying to determine a specific cutoff SUV for a population to predict recurrence is not appropriate. With these inflammatory changes so prevalent, it is imperative for the reading nuclear medicine physician and treating physician to review the images to define the solid and inflammatory components and their corresponding SUV in relationship to the distribution of the treatment to minimize the possibility of false-positive readings. It is therefore to our advantage to have scans read by a single physician for consistency in reporting.
Further refinement of this data is vital as quantification with SUVmax oversimplifies the evaluation of tumor response and sometimes results in inconclusive or inaccurate diagnosis. Currently investigators are trying to extract more detailed spatial–temporal PET/CT features [42] that comprehensively characterize the whole tumor’s intensities and distributions, spatial variations (texture), and shape properties which may become better predictors of treatment response. In addition, novel radiotracers are in development. The use of fluorodeoxyglucose (FDG) as the radiotracer of choice for PET scanning has been recently challenged. Investigators have looked at l–s-methyl-11C methionine (MET) PET. While MET has been shown to have decreased in inflammatory lesions as compared to FDG, a small series from Japan in the SBRT setting showed very little difference between the two tracers [43]. 18F -3′-fluoro-3′-deoxy-l-thymidine (FLT) [44], a nucleoside reverse transcriptase inhibitor, has been suggested to be superior to that of FDG in detecting cellular and proliferative responses to chemotherapy; the role of FLT-PET as a surrogate of response following stereotactic body radiotherapy (SBRT) for lung cancer remains unexplored. Similar data is available for patients with liver metastases and lung metastases.
Normal Tissue Response to SBRT
Normal tissues that function primarily as parallel normal tissues are made up of predominately structurally defined functional subunits (FSUs) (e.g., peripheral lung, liver, kidney). Serial functioning normal tissues, characterized by a chain of function, are composed of primarily undefined FSUs (e.g., gastrointestinal mucosa, trachea, spinal cord, bronchus). Parallel organ toxicity is mostly related to volume irradiated (e.g., mean dose, volume treated to a threshold dose such as V20), whereas serial organ toxicity is mostly related to the maximum dose delivered to that tissue. Although useful, this distinction between serial and parallel functioning organs is likely too simplistic, and most normal tissues likely have both parallel and serial functionality. This has been demonstrated in elegant rat model experiments of spinal cord tolerance to radiation therapy by van der Kogel et al. [45]. The rat spinal cord tolerance to radiation was found to be dependent on the volume irradiated and the spatial distribution of dose. The gray matter of the cord was found to be most resistant, whereas the lateral white matter was most sensitive.
As the volume of normal tissues irradiated to high doses with SBRT is generally less than the volume of normal tissue irradiated with conventional fractionation, for normal tissues that are primarily parallel functioning, if the volume irradiated is low enough, the risk of toxicity may be extremely low despite delivery of very high doses to a small volume. However, for serial organs that are in close proximity to tumors, even a small volume irradiated to a high dose may lead to irreversible toxicity. Thus, SBRT should be used cautiously for tumors adjacent to serial functioning organs such as the esophagus or spinal cord. An increase in the number of fractions may improve the therapeutic ratio and should be considered in place of hypofractionated SBRT in this situation.
With a dramatic increase in the fraction size and total dose with SBRT, the repair mechanisms may not be initiated as they would at a lower dose per fraction, potentially leading to permanent damage to the normal tissue within the high-dose volumes and/or unpredictable effects outside the high-dose volume. Given the potential for normal tissue injury and that the volume tolerance of normal tissues to such high doses per fraction is unknown, most SBRT has been applied to small tumors (<5 cm) in which the volume of normal tissue around the tumor is small. SBRT is being investigated for larger tumor volumes [46], and it is hopeful that clinical data will eventually be obtained to provide guidance to what the dose–volume toxicity relationship is for organs irradiated with inhomogeneous doses from hypofractionated fractionation schemes.
As very steep dose gradients are used with SBRT with rapid falloff of dose in surrounding normal tissues, not only is there substantial variability of dose throughout normal tissues, but also there is substantial variability in the dose per fraction. Thus, when dose–volume characteristics of a normal tissue associated with toxicity risk are investigated, correction for the variability in dose per fraction should be considered. Unfortunately, accurate knowledge of the α/β ratio for normal tissues is lacking. Clinical trials of novel fractionation regimens are required to confirm outcomes and toxicities associated with the many different fractionation schemes used in SBRT. Under each of the clinical sections, additional data will be presented based on complications to the bowel, chest wall, rib, liver, and brachial plexus.
Immobilization
Overview
Given the sensitivity of highly conformal SBRT plans to setup uncertainty and organ motion, reduction of geometric uncertainties and organ motion is important. The choice of patient position and immobilization may impact setup error, organ motion, as well as intrafraction motion secondary to patient discomfort. For example, prostate motion due to breathing is reduced in the supine position compared with the prone position [47, 48]. The different immobilization devices and strategies that have been used for SBRT are described below.
Spinal Tumor Immobilization
Similar to intracranial SRS with a cranial halo secured to the skull with transcutaneous pins, rigid fixation systems have been used for paraspinal and spinal SBRT. Hamilton in 1995 immobilized the spine for SBRT with rigid skeletal fixation above and below the involved segments. With this system, excellent accuracy, with less than 2-mm offsets, was observed [3]. However, such an approach is invasive, and avoidance of invasive fixation systems is desirable to minimize risks.
Noninvasive stereotactic systems using a frame, with or without implanted fiducial markers, have been used for paraspinal tumor SBRT with accuracy within 2 mm [49]. Optical-guided three-dimensional ultrasound has also been used for spinal SBRT to ensure that the patient does not move during radiation [50].
Body Tumor Immobilization
Nonrigid fixation has been performed for SBRT with specialized body frames that have fiducial systems attached to the frame and a device to control respiration using abdominal compression [4]. Abdominal compression using such frames has been found to reduce diaphragm caudal–cranial motion to less than 5–10 mm in most patients. Reproducibility of target positioning using these frames has been reported to be better than conventional immobilization systems, with positional deviations of lung cancer and liver cancer position less than 10 mm in 98 % of patients [4, 51, 52].
An option to these specialized body frames that facilitates guidance is to not use the frames for guidance, but to image internal anatomic references, such as bones near the target or the soft tissue tumor itself, to define the treatment coordinates. Repositioning and repeat verification imaging are required to ensure the patient was moved to the correct position. A variety of immobilization devices can been used with this strategy, as long as they keep the patient immobilized and preferably are (relatively) comfortable to minimize intrafraction patient motion.
Assessment of Breathing Motion
Organ motion due to physiologic functions during a radiation treatment fraction can be substantial. For example, the liver can move up to 5 cm in the caudal–cranial direction during free breathing [53] causing motion of the upper abdominal and lower thoracic cavity. As this motion can result in alternations in target and normal organ volume definitions, PTV margins, and the entire dose distribution, interventions to reduce the impact of intrafraction organ motion are required for many SBRT patients, to facilitate dose escalation and to reduce the volume of normal tissue irradiated.
Strategies to compensate for breathing motion include the use of abdominal pressure, voluntary shallow breathing, voluntary deep inspiration, voluntary breath holds at variable phases of the respiratory cycle, active breathing control (ABC), gated radiotherapy, and real-time tumor tracking. Although voluntary breath holds may be beneficial for some patients, there is potential for leaking air and patient error, particularly for patients with lung disease where usually less than 30 % of patients can even tolerate this approach. ABC refers to organ immobilization with breath holds that are controlled, triggered, and monitored by a caregiver. In approximately 60 % of patients with liver cancer, ABC was used successfully, with excellent reproducibility of the liver relative to the vertebral bodies within the time period of one radiation fraction (intrafraction reproducibility, σ, of the liver relative to the vertebral bodies: 1.5–2.5 mm) [54, 55]. However, with ABC, from day to day the position of the immobilized liver varies relative to the bones (interfraction reproducibility, σ 3.4–4.4 mm), providing rationale for daily image guidance when ABC is used for liver SBRT although treatment time needs to be kept reasonable as patients with poor performance status have increased treatment uncertainties and all patients have increased uncertainties with treatment times >10 min due to fatigue, muscle relaxation, or peristalsis.
Gated radiotherapy, with the beam triggered to be on only during a predetermined phase of the respiratory cycle, usually refers to the use of an external surrogate for tumor position (as opposed to direct tumor imaging) to gate the radiation. This can be used to reduce the volume of normal tissue irradiated. Changes in baseline organ position can occur from day to day [56], and thus image guidance is important to avoid geographic misses, especially in the setting of SBRT. One concern regarding gated radiotherapy has been brought to light by a French prospective phase III trial presented at ASTRO 2012 [57]. This trial was testing the benefit of gating in locally advanced lung cancer setting and surprisingly demonstrated an increase in radiation pneumonitis in patients treated with gating. The manuscript will hopefully shed more light on other variables that led to this paradoxical finding but more importantly should put caution in inexperienced users of this technology.
Tumor tracking is another approach to reduce adverse effects of organ motion. An elegant real-time tumor tracking system consisting of fluoroscopic X-ray tubes in the treatment room allowing visualization of radiopaque markers in tumors was first described by Shirato et al. The linear accelerator is triggered to irradiate only when the marker is located within the planned treatment region [58]. As an alternative to turning the radiation beam off when the tumor moves outside treatment region, multileaf collimators, the couch position, or the entire accelerator on a robotic arm may move with the tumor to ensure adequate tumor coverage at all times (e.g., CyberKnife image-guided radiosurgery system; Accuray, Sunnyvale, CA). The latter system uses dual orthogonal fluoroscopy tubes to track radiopaque markers in or near the tumor at a preset frequency.
There are advantages to gating, breath hold, and tracking in exhalation phase of the respiratory breathing cycle versus inhalation. These include the fact that exhalation tends to be more reproducible and is longer than inhalation, so that treatment during exhalation reduces duty time. However, in certain situations, there may be rationale for breath hold, gating, or tracking during inhalation. For example, for lung tumors and/or tumors adjacent to the heart, inhalation will reduce the density of the lungs and/or may move critical structures away from the target volume.
Other approaches to minimize respiratory and non-respiratory organ motion include maintaining the same preparative regimen prior to each treatment and ensuring comfortable immobilization and short overall treatment time to reduce patient movement due to discomfort and physiologic change in organs such as stomach filling. Another general intervention is patient feedback, either auditory or visual. Ideally, feedback would be from direct tumor imaging; however, feedback from imaging of adjacent organs, spirometry, nasal flow monitoring, external marker position, or optical monitoring of body contour are often more practical options. If indirect measures of organ position are used, confirmation for an individual patient that the indirect measure is indeed directly related to organ position is mandatory.
Treatment Planning
Overview
As dose gradients are steeper and doses are higher with SBRT than with conventional radiation therapy, the consequences of error in tumor delineation, errors introduced by dosimetry, and geometric uncertainties may be more deleterious. Thus, all aspects of treatment planning that are important in conformal radiation planning are even more crucial in SBRT, especially for tumors in close proximity to critical normal tissues, where a systematic error could lead to permanent serious toxicity if the normal tissue planned to be spared from radiation is irradiated to the high doses planned for the tumor.
Imaging at Simulation
At the time of simulation, patient positioning and the imaging modality (CT, MRI, PET/CT), resolution (e.g., CT thickness), and phase of contrast (e.g., arterial IV contrast for HCC or how to give IV contrast during a four-dimensional scan for a tumor near a major vessel) must be chosen carefully. The patient treatment position should be kept the same as scanning position. Due to the relatively long treatment time, a comfortable patient position is preferred to reduce the intrafraction motion. Since more beams are used at SBRT planning and they need to be separated maximally in space, beam arrangement should be considered during patient simulation. For example, for lung cancer patient, lifting both arms allows beam arrangements in 360°. The report of AAPM Task Group 101 [59] has recommendations about the CT thickness and scanning length. In this report, 1–3-mm CT slice thickness was recommended and a typical CT scan for planning purpose should cover the tumor site plus 5–10 cm in both superior and inferior directions. If non-coplanar beams are used, 15 cm is recommended to be extended in both superior and inferior directions. Motion must be considered at this time, as breathing introduces artifacts in the tumor definition, normal tissue definition, and resultant errors in TCP and normal tissue complication probability (NTCP). Furthermore, if motion is not considered, there is potential for a systematic error from the time of simulation to the time of treatment to occur. Motion due to breathing is largest for tumors near the diaphragm (i.e., the upper abdomen and the lower thorax). One method to account for motion is to eliminate it, for example, with a breath-hold scan. Diagnostic breath-hold scans are often obtained in the inhale position, which may not correspond with the treatment position (e.g., exhale breath hold). An effort to conduct all imaging to be used for planning with the patient in the same position, with the same phase of breath hold, is required. If breath hold is not possible or if reproducibility of this breath hold cannot be documented, reduction of breathing motion may help reduce the negative impact of breathing motion. However, even with a small range of breathing motion, errors in tumor and normal tissue volumes may occur, resulting in a geographic miss or excessive toxicity. An option to breath-hold imaging is to obtain a four-dimensional imaging data set. From this, any position could be used for planning and image guidance [56]. Planning on the exhale data set with asymmetric PTV margins is an option [60], as is planning using the mean tumor position. The phase of the breathing cycle in which the patient is planned should correspond with the phase of breathing cycle used for image guidance and treatment.
Target Volumes
A decision has to be made regarding whether a margin is required for microscopic disease risk or the clinical target volume (CTV) margin. Although in many SBRT series, no extra margin for CTV has purposely been added but due to the less steep falloff in dose when treating the body as compared to the brain, there is always a dose gradient from the prescription dose to a “microscopic dose” [61]. In addition, the additional margin added for motion and setup error likely overlaps with the needed margin extension. This added margin may also be significantly different based on histology where subclinical disease distant from the index lesion is more prevalent such as in breast cancer. At Princess Margaret Hospital in Toronto, for our present SBRT liver cancer studies, a 5-mm margin around the GTV within the liver is used to define the CTV. The planned minimum dose to the CTV PTV is 27 Gy in six fractions, while the dose to the GTV may be as high as 60 Gy in six fractions at the periphery. Careful radiologic–pathologic studies accounting for organ deformation and shrinkage are required to determine whether the use of a CTV margin is required or not.
Finally, appropriate PTV margins must be used to ensure that the actual planned doses are delivered to the tumor. The PTV margins must consider setup uncertainty and internal organ motion. Individual institution setup uncertainty data should be used if available. Individual patient internal organ motion is preferable to using population-based respiratory motion. Of note, the classic PTV margin papers on PTV margin determination, such as that published by Van Herk et al.[62], do not apply to very tightly conformal plans delivered in a few fractions, such as those used in most SBRT cases. Thus, the margin recipes that are used for conventional radiation planning may be inappropriate for SBRT plans. Modeling has demonstrated that when PTV margins are too small, higher doses must be delivered for the same TCP [63].
Planning
Often, many beams or dynamic conformal arcs are used to develop a highly conformal SBRT dose distribution. In principle, a great number of beams maximumly separated in space lead to more conformal plan; however, the beam optimization should consider target size and irregularities, OAR avoidance, the length of beam path, the treatment delivery time, and patient safety. The energy of 6–10 MV is preferred due to the neutron contamination and the larger penumbra of high-energy photon beam. Limited high-energy beam may be allowed if the beam path is larger than 10 cm. Sometimes, non-coplanar beams or arcs are used if required to reduce the dose to normal tissues. For example, 8–12 beams may be used for a typical lung SBRT plan. Non-coplanar beam setup should be used carefully to avoid the patient, couch, and gantry collision. Although the more beams are preferred in SBRT planning, the resultant longer treatment time has to be considered in practice. One strategy to obtain highly steep dose gradients at the edge of the PTV is to close the aperture of the beams to the PTV or less, not leaving a gap for penumbra as usually done for conformal radiation therapy. When enough beams are summed together, the PTV may be covered by a lower isodose such as 65 % that is often at the steepest part of the dose gradient. Choosing such a low prescription isodose line may have some theoretical disadvantages since a significant rind of normal tissue is in the PTV. Investigators at Fox Chase Cancer Center and at the University of Maryland use a 3-mm distance between the beam aperture and the PTV [64]. The prescription isodose line between 70 and 80 % is reasonable if the rind of normal tissue inside the PTV is small or can tolerate this high dose. Resultant high doses/hot spots occur in the center of the PTV, perhaps giving the highest dose to the center hypoxic volume (although the potential benefit of this is unproven). Of note, the use of many beams for SBRT is not mandatory. For a peripheral liver tumor, three to five beams may be used to reduce the overall radiation path length through the liver. The use of segments within the beams can adjust the dose distribution to ensure that the hot spot is within the GTV while reducing the overall integral dose.
As the adverse effects of dosimetric errors are most pronounced with SBRT, appropriate methods to consider corrections for heterogeneities should also be used (see next section).
Typical prescription doses for SBRT range from 5 Gy in ten fractions to 20 Gy in three fractions. One to five fractions are most often used, with a dose per fraction usually greater than 6 Gy. The common feature to most of the SBRT fractionation schemes is that they are biologically potent. Multiple-fraction regimens have some radiobiologic advantages to single-fraction SBRT. Clinical data is not available to provide guidance for the most appropriate fractionation for each clinical scenario. However, when the PTV is in very close proximity to normal tissues that function serially, it is reasonable to consider prolonging fractionation to minimize the risk of toxicity to the serial function normal tissue.
It is a challenge to relate the prescribed dose to TCP, as inhomogeneous doses are generally used and various delivery and verification techniques are utilized. For a moving target not treated with image guidance, the actual delivered minimal dose to the tumor may be lower than the prescribed dose. Furthermore, there is heterogeneity in patterns of prescribing dose. One method to account for inhomogeneous dose distributions is to use equivalent uniform dose (EUD) to tumor for reporting [65]. Of course, accounting for individual patient motion in the dose distribution or eliminating motion would help ensure that the reported doses better reflect delivered doses as well. Unfortunately, it is not possible to account for motion in current commercially available planning systems.
Plan Evaluations
All conventional 3D plan evaluation methods apply to SBRT plan, but three criteria are specially considered: dose conformality, dose gradient, and dose heterogeneity.
Since SBRT delivers high dose (often >5 Gy per fraction) in a small number of fractions (≤5), high-dose conformality is required to minimize the dose to the normal tissue. A frequently used conformality index is defined as the ratio of volume receiving prescription dose to the PTV. A perfect plan should have conformality index equal to 1, i.e., PTV is covered by 100 % of prescription dose and no normal tissue receives the prescription dose or above. This is not realistic. Usually the conformality index of 1.3 is achievable. The index could be larger for smaller target or irregular target. In current or recently closed RTOG protocols of lung SBRT [10], for the target with maximum dimension between 2 and 7 cm, the conformality index was required to be less than 1.2, and the plan was considered as minor deviation with index between 1.2 and 1.4 but there is no good data to suggest complications are higher with a poor conformality index. As with similar data with intracranial SRS, target volume and proximity to critical structures usually trump the effect of conformality.
A sharp dose gradient out of PTV is desirable for SBRT plans. The dose gradient can be evaluated by how much %/mm or ratio of volume receiving 50 % of prescription dose to the PTV that was used in RTOG protocols of lung SBRT [10]. Usually a sharp dose-off isotropically is preferred although this can be limited to less degrees of freedom with non-coplanar fields, but the dose gradient can be larger in one direction if close to a critical structure.
The dose heterogeneity is probably not as important as the above two parameters, although it needs attention. To increase the plan conformality index and dose gradient usually increase the dose heterogeneity. The dose hot spot should be within the PTV, and the best place is the center of the PTV.
The dose constraints to critical organs are different with what are used in the conventional therapy. So far the dose tolerance is still immature and the dose to organ at risk should be evaluated carefully according to the peer-reviewed publications or protocols.
Treatment Planning System Considerations Related to Heterogeneity Corrections
The dose calculation heterogeneity correction is desirable for SBRT planning, especially for lung or spine SBRT. The treatment planning system (TPS) should be carefully evaluated to see if it is suitable for SBRT planning or specific site. Pencil beam algorithms have been discouraged for tumor sites surrounded by low-density tissue [59]. It has been reported that there was significant dose difference in the target periphery between pencil beam algorithm and superposition convolution algorithm for the lung SBRT [59]. Although the Monte Carlo simulation is the most accurate dose calculation algorithm, it may not be widely available for commercial TPS and take more time for dose calculation. The convolution superposition algorithm is more suitable considering the accuracy and calculation time is rather similar to Monte Carlo-generated plans.
Early in the North American experience with lung cancer, heterogeneity corrections were not used because tissue density correction dose algorithms were not widely available. In the absence of heterogeneity corrections, tumors completely surrounded by air experience a dose build-up effect due to the loss of electronic equilibrium, such that the periphery of a tumor is under-dosed, and a higher dose is required to achieve optimal cell kill [66]. Tumors that lack this air–tissue interface, such as tumors abutting the chest wall or central tumors, may have artificially high doses (“hot spot”) delivered to the PTV, due to dose being delivered through low-density air using very long path lengths. Xiao et al. [67] applied heterogeneity corrections to the original treatment plans of 20 patients from RTOG 0236. They observed an increase in isocentric dose in all patients following the application of heterogeneity corrections. However, the mean volume of PTV receiving 60 Gy decreased from 95 to 85 %, and dose to 95 % of the tumor decreased by an average of 4.7 Gy [67]. Based on these findings, the use of heterogeneity corrections would be associated with larger hot spot doses but worsened overall tumor coverage, potentially leading to increased complications due to the high-dose areas and higher potential for failure due to underdosing parts of the tumor. This led the investigators to conclude that the RTOG standard dose should in fact be 18 Gy per fraction delivered to the 95 % isodose, for a total dose of 54 Gy. Notably, these patients all had peripherally located tumors.
Investigators at the University of Maryland repeated this work on patients with peripherally and centrally located lung tumors. This study shows that dose to the internal target volume (ITV) increases with the application of heterogeneity corrections and the effect was even greater with centrally located tumors and larger. In centrally located tumors, where more lung parenchyma is located between tumor and body surface, intervening tissue density is overestimated. When this is corrected, dose to the tumor increases, an effect observed in this study. This too is true of larger tumors, where increased beam widths traverse more parenchyma prior to reaching the PTV. Given the data above, the inclusion of central tumors in this sample may in part explain why the application of heterogeneity corrections led to an increase in D 95 in this study, versus a drop in D 95 as observed in the paper by Xiao et al. [67], in which central tumors were excluded.
Without heterogeneity corrections applied to treatment plans, a higher dose must be prescribed to achieve adequate dose to the tumor, which also results in a higher dose to the surrounding parenchyma. This increases the risk of adverse outcomes, which may help to explain the increased complications seen in the initial Indiana experience. Based on these results, the use of heterogeneity corrections allows the prescription of a lower total dose and dose per fraction, with a comparable dose to the tumor. This less aggressive dose prescription may allow safer yet equally efficacious treatment of NSCLCs.
Image Guidance
Overview
Image guidance at the time of treatment can improve setup accuracy, reduce PTV margins, and irradiate the volume of normal tissue, facilitating safe dose escalation.
Traditionally, surrogates for the target have been used in guiding the placement of treatment fields. For example, skin marks for patient position are routinely used for initial patient setup in practice. For some clinical situations, the use of skin marks to align the patient can be done with high precision. However, in most body tumors, the internal structures cannot be accurately localized with the use of skin marks. It has been reported the random setup error is up to 6.6 mm for pancreatic cancer patients receiving SBRT based only on body marker. The use of bony anatomy with electronic portal imaging is another standard practice in radiation therapy. However, for many clinical situations, the bony anatomy is not well correlated with the internal tumor position. Options for locating internal anatomy include the use of implanted radiopaque fiducial markers as surrogates for the target, tissues adjacent to the tumor, or the tumor itself. Fiducial markers may also be used to measure organ motion and/or track/gate the beam.
Many of the published reports of SBRT have been on patients treated with conventional linear accelerators. However, more specialized treatment units are now available with the potential to allow soft tissue image guidance and reduced PTV margins. Examples of such systems include a lightweight and robotic linear accelerator (CyberKnife; Accuray, Sunnyvale, CA) and modified linear accelerators to allow image guidance including accelerators such as Novalis (BrainLAB, Inc., Westchester, IL), Synergy (Elekta Oncology, Stockholm, Sweden), TrueBeam and Trilogy (Varian Medical Systems, Palo Alto, CA), Artiste (Siemens, Concord, CA), and TomoTherapy (Madison, WI).
Image-Guidance Strategies (Pretreatment)
Imaging at the time of treatment can be used for localization for guidance, verification, and also as a quality assurance (QA) tool that is evaluated by the treating physician prior to each treatment. Verification that the appropriate dose is actually delivered is also important for SBRT. Soft tissue imaging at the time of treatment can allow a measurement of the impact of geometric uncertainties (such as organ deformation) at the time of treatment. When image guidance and repositioning are used, imaging after repositioning should be used to ensure the positioning moves were made in the correct direction.
When repositioning moves are required due to changes in internal organ position, replanning is not routinely conducted. However, when substantial changes in organ position and or tumor size or breathing pattern occur, the dose delivered may be altered due to changes in radiation path lengths and position of organs with difference heterogeneities. The magnitude of dosimetric differences due to such changes should be studied to better define in which situations replanning may be of benefit. In addition, when soft alignments are utilized, the treating physician should also ensure the volume visualized on the volumetric imaging is similar to the ITV generated on the 4D CT as differences may be related to different breathing patterns from day to day and may not improve alignments.
Two-Dimensional Image-Guidance Equipment
Orthogonal megavoltage (MV) portal films and more recently images from electronic portal imaging devices (EPIDs) have traditionally been used for image guidance and may be appropriate for targets adherent to the bones. MV images of large fields of view can be obtained with 2–8 monitor units (MUs) per image. These images not only can guide therapy but also can verify the shape and orientations of the treatment fields. If radiopaque fiducial markers are inserted in or near the tumor, the fiducial markers themselves may be used for guidance. Other alternatives for guidance include using surrogates that are in close proximity to the tumor, for example, the diaphragm as a surrogate for liver tumors [68, 69]. An example of an anterior–posterior (AP) MV image for liver cancer guidance, in which the diaphragm is used for cranial–caudal positioning.
Due to the low contrast of MV radiographs and the doses delivered with repeat MV imaging, orthogonal kV radiographs and kilovoltage (kV) fluoroscopy have also been used for image guidance of tumors and/or fiducial markers, either immediately prior to each radiation fraction [68, 69] or throughout radiation delivery [58]; kV X-ray tubes may be ceiling or wall mounted or attached to the linear accelerator.
With both MV and kV orthogonal imaging, alignment tools registering the images to digitally reconstructed radiographs (DRRs) can improve the accuracy and efficiency of image matching to determine the offsets in position. Such alignments tools include template matching tools based on therapists’ visualization of anatomy and/or automated image registration of the region of interest (e.g., mutual information). Decision rules including tolerance levels for repositioning must be integrated with the overall system.
Three-Dimensional Volumetric Image Guidance
Technological advances allowing volumetric imaging allow image guidance immediately prior to treatment using the tumor or a soft tissue organ in close proximity to the tumor for guidance, rather than the bony anatomy. Advantages of volumetric imaging systems include that adjacent normal organs can also be visualized for more accurate avoidance of critical structures. Some of these volumetric imaging techniques can also measure tumor motion due to breathing.
Since the soft tissue can be visualized with three-dimensional volumetric images, it is possible to set up the patients based on the tumor target or organ at risk if which has more priority during the treatment. The pitfall of this strategy is that it may introduce the large setup uncertainty for target which has respiratory-induced motion. Currently, the CT scanner is fast enough to image the moving target on one breathing phase. The CBCT is slow and the moving target is more similar to internal moving target (ITV) on CBCT images. The in-room CT is actually a CT scanner and the moving target similar to one breathing phase. If the patient is aligned based on the target between planning CT and CBCT/in-room CT, the perfect target match may introduce the errors due to the difference in breathing phases between planning CT and CBCT/in-room CT. The correct strategy is to align the patient based on the bony structure and make sure that the target acquired through CBCT/in-room CT is within the PTV on the planning CT.
In-Room CT
The placement of a diagnostic CT scanner in the treatment room with a known geometric relationship to the linear accelerator is one approach for volumetric imaging immediately prior to treatment with the patient in their immobilization device. Uematsu et al. have used this approach to treat liver and lung cancers with SBRT. Uematsu et al. reported that with repeat CT for frameless head and neck cancer radiotherapy, there was a geometric vector error ranging from 0.1 to 0.9 mm [70]. Multiple manufacturers have developed products of this type, including Siemens’ Primatrom, Mitsubishi’s accelerator in combination with a General Electric CT scanner, and Varian’s ExaCT targeting system [71–73]. All systems place the CT scanner in close proximity to the linear accelerator, allowing the couch to be moved from the imaging position to the treatment position. The CT scanner gantry is often translated during acquisition to minimize couch motion. Accuracy has been reported to be under 0.5 mm [72], and it has been reported to be improved with fiducial markers from 0.7 to 0.4 mm [71].
Advantages of in-room CT include that state of the art, diagnostic-quality CT can be used for optimal image quality and robustness. Disadvantages of this system are that the imaging and treatment isocenters are not coincident. Accuracy of motion from the CT scanner gantry, the accelerator couch, and the coincidence of the CT and linear accelerator isocenters needs to be verified. Limiting patient movement between imaging and treatment (e.g., couch retraction <1 mm) should improve setup accuracy; however, organ motion between imaging and delivery may occur.
kV Cone Beam CT
Jaffray et al. [74] first described the concept of cone beam CT (CBCT) for image-guided radiation therapy in 1997. CBCT refers to combined kV X-ray imaging and MV radiation delivery in one integrated gantry-mounted system. Advancements in large-area flat panel detector technology facilitated volumetric imaging to be acquired in a single rotation of the linear accelerator gantry. Planar kV images projections are obtained as the gantry rotates about the patient on the linear accelerator table, over 30 s to 4 min. CBCT three-dimensional volume reconstruction images may then be obtained for position verification or for image guidance (see Fig. 13.2). Geometric calibration methodologies for CBCT systems [75] and quality assurance recommendations [74] have recently been proposed.
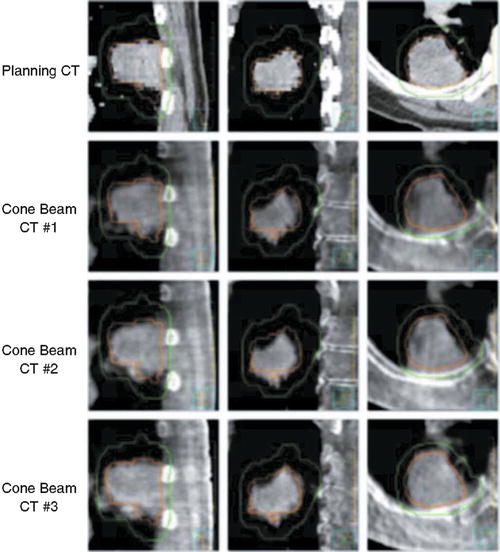

Fig. 13.2
Planning CT and kV cone beam CT from each lung cancer SBRT fraction. GTV from the planning CT scan is shown in orange and PTV in green
Doses delivered to obtain CBCT scans typically range from 0.5 to 2 cGy. Two vendors have developed kV CBCT systems: Elekta Synergy and Varian Trilogy and TrueBeam. In addition to providing volumetric imaging for verification and guidance, these systems have the ability to be used for real-time kV tracking; the latter application has not been used clinically.
Similar to in-room CT, artifacts may occur with kV CBCT reconstructions due to high Z structures such as surgical clips, hip prostheses, and dental fillings. Methods to reduce these artifacts have been developed.
MV Cone Beam CT
CT imaging using MV beams has also been explored for more than 20 years [76, 77] and has also been made possible with advances in portal imaging technology. Advantages of MV cone beam CT are that the treatment MV beam is used to obtain the imaging, requiring less modification to the linear accelerator; the electron density estimates for treatment planning are accurate; and there is no high Z artifact that is associated with kV imaging.
MV cone beam CT has been used to aid in lung cancer SBRT, as described by Nakagawa et al. in 2000. MV CT-aided lung SBRT was used for treatment of 22 lung tumors [78].
Pouliot et al. recently reported the feasibility of acquiring low-exposure megavoltage CBCT. Phantom and pig cadaver head and neck images were acquired using a linear accelerator dose rate of 0.01–0.08 MU per image, for a set of 90–180 projections, acquired in 1–2° increments over 45–60 s. MV cone beam CT scans were obtained with doses of approximately 5 cGy. Despite the low efficiency of this system, visibility of high-contrast structures, such as air and bone, was reasonable [79].
MV TomoTherapy
MV TomoTherapy combines tomographic scanning capabilities, from a conventional CT detector, with a linear accelerator mounted on a rotating gantry. Simpson described the initial development of an MV CT scanner for radiation therapy in 1982 [76]. More recently, the TomoTherapy treatment platform has become available for image guidance and verification. The MV treatment beam is used to obtain imaging, with a lower energy, 3.5 MV instead of 6 MV. Computer-controlled multileaf collimators, also on the rotating gantry, have two sets of leaves that open and close to modulate the radiation beam while the couch advances the patient through the gantry bore, for helical intensity-modulated radiation therapy (IMRT).
Similar to MV cone beam CT, there are no high Z artifacts with MV TomoTherapy.
Image-Guidance Strategies (During Treatment)
Real-Time Tumor Tracking
Real-time tumor tracking while the radiation beam is on is another approach to reduce adverse effects of organ motion. An elegant highly integrated tracking system consisting of four ceiling-mounted fluoroscopic X-ray tubes and four floor-mounted flat panel imagers in the treatment room allowing visualization of radiopaque markers in tumors was first described by Shirato et al. [58]. This system has a temporal resolution of 30 frames per second and a precision of 1.5 mm. The linear accelerator is triggered to irradiate only when the fiducial marker is located within a predefined volume. This system has been used for image-guided radiation therapy of lung, liver, and paraspinal malignancies.
As an alternative to turning the radiation beam off when the tumor moves outside the treatment region, multileaf collimators, the couch position, or the entire accelerator on a robotic arm may move with the tumor to ensure adequate tumor coverage (e.g., CyberKnife image-guided radiosurgery system). The latter lightweight (330 lb) linear accelerator mounted on a robotic arm uses 6 MV, 5- to 60-mm collimators, and a dose rate of 300–400 MU per minute, combined with dual orthogonal fluoroscopy tubes to track radiopaque markers in or near the tumor at a preset frequency. When the beam is on, infrared external surrogates are continuously monitored, while the internal anatomy is monitored every few seconds with kV imaging. The external surrogates are used for determining the breathing model, and the model is updated based on the X-ray data obtained every few seconds. The robotic linear accelerator responds to motion by moving to an appropriate position, within a range of ±10 mm x, y, z and ±1° pitch and roll, ±3° yaw. This system was found to have a 0.3-mm accuracy when tested in phantom studies. Disadvantages of this system include the need for fiducial markers, long potential delivery times (up to 90 min), lack of suitability for large tumors with motion more than 10 mm, and highly inhomogeneous dose distributions.
Another system (Novalis: BrainLAB, Heimstetten, Germany) also acquires kV orthogonal images and matches the images to DRRs obtained from the planning CT. The imaging axes are not coincident with isocenter, and a translation of patient position is required between imaging and treatment. Accuracy of this system has been reported to be within 3 % and 3-mm distance to agreement.
Recently, wireless transponders and infrared cameras to track tumors have also been proposed as an imageless localization system (Calypso Medical Technologies, Seattle, WA).
SBRT Patient Safety
Since SBRT gives very high dose and uses much smaller planning margin than conventional therapy, more caution must be given to avoid catastrophic dose delivery. The patient safety has got more and more attention in radiation therapy society. Recently, the ASTRO SRS/SBRT white paper was published to address SRS/SBRT quality and safety considerations. This chapter presented the personnel requirement and training for radiation oncologist, medical physicist, medical dosimetrist, radiation therapist, and administrator; presented the technology requirements of simulation, planning, and localization; and addressed the SRS/SBRT system acceptance and commissioning.
The report of AAPM TG101 also has recommendations for SBRT patient safety issue. It recommends that at least one qualified medical physicist presents during first fraction of SBRT treatment and the qualified medical physicist should be available for the following fractions. It also recommends that the radiation oncologist should approve the image guidance before each fraction treatment. The therapist should be well trained for the SBRT procedure.
Equipment Quality Assurance
The equipment of SBRT mainly include the linac system and imaging system. The QA frequency is daily, monthly, and annually. Usually the radiation therapist performs the daily QA and the qualified physicist reviews and approves it, and the qualified physicist performs the monthly and annual QA. Apparently SBRT linac has tighter tolerance than the linac only for conventional therapy. The report of AAPM Task Group 142 [80] published recommendations for SRS/SBRT linac QA. The main recommendations about accuracy tolerance are summarized here. It recommends that the laser, the couch movement indictor, collimator size indicator, collimator rotation isocenter, couch rotation isocenter, gantry rotation isocenter, and coincidence of radiation and mechanical isocenter have to be within 1 mm. The couch rotation indictor has to be within 0.5°. The imaging system includes kV/MV imaging and kV/MV cone beam CT. It recommends that the imaging and treatment coordinate coincidence should be within 1 mm. For cone beam CT, the geometric distortion should be within 1 mm, and the contrast, spatial resolution, HU constancy, uniformity, and noise should agree with the baseline.
Clinical Outcomes
The next several sections will review the most common clinical sites where SBRT/SABR is delivered most commonly where pertinent issues related to patient selection, simulation, treatment planning, and morbidity specific to that clinical site. This data should be used with caution when extrapolating to other locations (i.e., primary liver versus metastatic liver since the background normal tissue is already partially injured) but will be good starting point prior to treating a unique site.
Stereotactic Ablative Radiotherapy for Lung Tumors
Lung cancer is the second most common cancer diagnosis and the most common cause of cancer death for both genders. In 2012, there was an estimated 226,160 new lung cancer diagnoses in the United States and 160,340 deaths [81]. Of the 15 % of patients diagnosed with early-stage lymph node-negative NSCLC, the 5-year relative survival is only 52 %. The current standard of care for these patients is surgery [82]. When a patient cannot tolerate or refuses surgery, radiation therapy is an excellent alternative with current literature suggesting outcomes following SBRT/SABR similar to surgery. For patients with severe comorbidities, it is important to understand what the natural history of early-stage disease is. Approximately 50 % of patients require treatment for symptoms at 1 year and on average 50 % die of lung cancer at 2 years without treatment [83].
Patient Selection
This approach is widely accepted as the current standard of care for peripheral T1 or T2 tumors or certain T3 tumors (i.e., invading the chest wall) which measure less than 5–7 cm without any nodal involvement. Central lesions may have a higher complication rate in comparison to peripheral tumors based on the initial phase II experience published out of Indiana [8]. In their single institution experience, “excessive toxicity” was reported for patients with tumors located within 2 cm of the proximal respiratory tree where the rate of grade 3 and above toxicity was significantly higher among patients with central tumors. This increase in toxicity may be related to dose and dose per fraction. Stephans et al. [84] reported the experience at Cleveland Clinic, which clearly illustrates the effects of the higher biologic effective dose (BED) on morbidity. Their institutional practice of SBRT initially used 10 Gy × 5 fractions and subsequently changed to the RTOG standard (18–20 Gy × 3) when RTOG 0236’s results were initially reported. The local control rates showed no difference between the two schemas, although they did experience a high rate of chest wall morbidity with the higher BED regimen (18 % versus 4 %, p = 0.028). In addition, follow-up from the initial Indiana experience treating central tumors reported lower grade 3 and higher side effects when >3 fractions were used as opposed to three fraction (26.3 % versus 7.7 %) [85]. However, caution is still indicated. Washington University [86] has completed a phase I study demonstrating 60 Gy in five fractions is safe for treating central tumors and is currently accruing to a phase II study. Many retrospective series also suggest a five fraction is safe in treating centrally located tumors [87]. In addition, a multi-institutional phase I/II [88] RTOG study is currently nearing completion using a similar fractionation schedule as the Washington University. Led by Videtic et al. [88] from the Cleveland Clinic, the RTOG has currently completed testing two less aggressive schemas (12 Gy × 4 fractions and 34 Gy × 1 fraction) in a randomized phase II trial, specifically looking at morbidity at 1 year as the primary end point as local control is expected to be 90 % in both arms (RTOG 0813). The less aggressive regimen will then be compared to the current RTOG standard which is a subsequent phase III trial. There is no lower limit of lung function based on pulmonary function tests that predicts poor outcomes [89]. One clinical entity that may have a higher risk of pulmonary injury is patients with interstitial pulmonary fibrosis. Onishi et al. [90] described 24 patients who died of fatal pneumonitis in the large multi-institutional experience. Seventeen of the 24 patient had IPF with 1 of the patients with usual interstitial pneumonia. The absolute risk of fatal pneumonitis for this cohort of patients in Japan is not known, but from Washington University described a 20 % risk of pneumonitis in patients with IPF. So a diagnosis of IPF is not a contraindication for SBRT but does appear to be at a higher risk of pneumonitis.
Treatment Planning
During the CT simulation, 3-mm slice thickness or less is preferred. IV contrast may be helpful for tumors close to the brachial plexus or for central tumors adjacent to vasculature. The gross tumor volume (GTV) is contoured on pulmonary windows, although soft tissue windows are helpful for tumors adjacent to the chest wall and central lesions. Contouring all the spiculations on lung windows is controversial and not uniformly performed. At the University of Maryland, only the bulky component of the spiculation is contoured (2-mm thickness) based on unpublished literature suggesting that it is uncommon that these radiographic findings correlate with clinically significant disease and is covered with subclinical doses in the area of dose falloff [61]. There are institutional preferences regarding the use of a margin for microscopic extension and creation of a CTV. In RTOG 0236, it was specified that the GTV was equivalent to the CTV. Assessment of respiratory motion and creation of an ITV is preferred to limit the PTV margins similar to SRS in the brain. The ITV can be assessed with a 4D CT scan or with fluoroscopy to track the tumor or a fiducial implanted into the tumor. A margin of 0.5 cm to create the PTV from the ITV is common. If an ITV is not created, population-based margins of 0.5 cm axially and 1.0 cm in the cranio-caudal directions were used historically in the Indiana experience and RTOG 0236. However, the use of patient-specific tumor motion is preferred. This can be performed by fusing maximum inspiration and expiration breath-hold scans to a free breathing CT, obtain a “slow” CT simulation, if 4D CT is not available. 4D CT has been demonstrated to result in smaller PTV volumes than any of the other described options [91]. If there is significant respiratory motion, some have advocated for managing this with abdominal compression or breath-hold maneuvers. The benefit of abdominal compression is likely to be greatest for lower lobe tumors. However, abdominal compression may increase tumor motion in some cases and should be considered on an individual basis and there are several studies which demonstrate increased failure with the use of abdominal compression and gating [92]. At the University of Maryland, a CTV margin of 3 mm is added based on the unpublished work from Fox Chase Cancer Center which evaluated 25 consecutive patients who underwent a lobectomy or sublobar resection for a curative procedure for tumors <3 cm in maximum dimension and also had a diagnostic CT scan for review. Based on a comparison of CT and microscopic pathology review, the entire microscopic extent of disease was covered when 3 mm was added to the maximum extent of the disease contoured on standard lung windows without chasing the spiculations in 95 % of the cases. An additional 3 mm was added for setup error based on obtaining a daily CBCT prior to each treatment.
Interfraction setup variation is reduced with image-guided radiotherapy (IGRT). This can be performed by matching fiducial location, which is vital to technologies which do not use volumetric IGRT, and/or creating a CBCT with the patient immobilized. These pretreatment images are compared to the simulation images and adjusted as appropriate. Fusion of the CBCT with the simulation CT using bony anatomy is recommended, as there is minimal change in the target centroid position relative to the skeletal frame [93]. The differences in patient position based on matching with bony anatomy versus soft tissue can be off by upwards of 8 mm on average but this is primary based on our medically inoperable patients who are likely to have emphysema and poorly functioning diaphragms [94]. Alignment using soft tissue can be performed if imaging acquired on cone beam imaging is similar to that generated by the 4D CT-based ITV. If the volumes do not match, then the treating physician needs to consider that the patient’s breathing pattern may be different from the day of the initial 4D CT. This difference may not be reflected accurately on the CBCT and alignment based on soft tissue may therefore be inaccurate potentially leading to a geographic miss. In this situation, a bony alignment is preferable and treatment can be delivered if the volume is safely within the PTV on the CBCT. If it is outside the volume, a repeat 4D CT is preferred.
At the University of Maryland, consideration of the accuracy of cone beam imaging is considered to be 2 mm such that a shift will be performed only if the shift is 3 mm or more based on the work from Princess Margaret Hospital [95].
Fractionation
There are two predominant fractionation schedules used in the United States: the Japanese approach (12 Gy × 4) [96] and the original Indiana approach developed by Dr. Timmerman (18–20 Gy × 3) [6, 8]. Outcomes and dose–response will be discussed under clinical outcomes. In the original Indiana series that led to RTOG 0236, dose is calculated without heterogeneity corrections. When these are applied, the dose is on average 10 % lower for the subset of patients treated on the study [22]. The prescription isodose line covering the PTV is usually 60–90 % depending on the block edge margin (2–3 mm, respectively) used on each beam’s eye view. Typically a minimum of 7–9 non-coplanar, non-opposing beams are required to obtain adequate dose falloff to minimize risk of morbidity (see Fig. 13.3). Plans are assessed by dose conformality similar to strategies utilized in the brain where the primary goal is to devise the most conformal plan to the PTV with the secondary goal to minimize the volume receiving 50 % of the prescription dose. The currently accruing RTOG protocols recommend that 95 % of the PTV be covered by the prescription isodose and 99 % of the PTV receives a minimum of 90 % of the prescription dose. A prescription isodose to PTV volume (PITV) ratio of 1.2 or less is recommended, except for tumors smaller than 1.5 cm as this constraint is difficult to meet. Additional RTOG recommendations are in place to limit toxicity to normal structures. This includes no more than 15 % of the PTV volume of tissues outside the PTV receiving more than 105 % of the prescription dose. The so-called intermediate dose spillage is limited by imposing criteria on the ratios of the 50 % prescription isodose volume to the PTV volume and the maximal dose at 2 cm from the PTV, which are dependent on PTV volume. This uniform dose falloff may be important especially in the parenchyma of the lung where subclinical disease may be present.
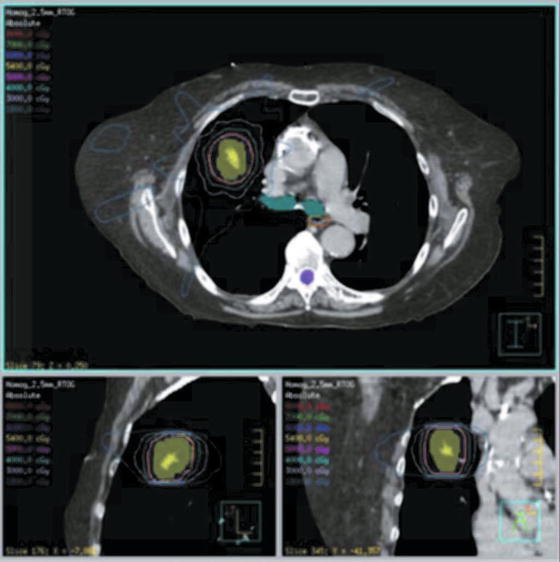

Fig. 13.3
SBRT dose distribution for lung cancer
Clinical Outcomes
SBRT/SABR has been most widely studied in primary and metastatic lung cancer. A summary of clinical data is shown in Tables 13.1 and 13.2 and some of the major studies are discussed in greater detail below.
Table 13.1
Selected results of primary non-small cell lung cancer treated with SABR
Reference (year published) | No. patients | Patient population (all N0 M0) | Total dose (no. fractions) | Median follow-up (months) | Local control | Regional control rate (%) | Distant metastasis rate (%) | Overall survival |
|---|---|---|---|---|---|---|---|---|
Uematsu (2001) [23] | 50 | 18 pts received 40–60 Gy prior to SABR | 50–60 Gy (5–6) | 36 | 94 % | 96 | 14 | 66 % at 3 years |
Wulf (2004) [30] | 20 | 10 % T1 | 30–37.5 Gy (3) | 11 | 95 % | NR | 25 | 32 % at 3 years |
50 % T2 | ||||||||
40 % T3 | ||||||||
Nagata (2005) [19] | 45 | 71 % T1 | 48 Gy (4) | 30 | 98 % | 93 | 20 | At 3 years: 83 % for T1 and 72 % for T2 |
29 % T2 | ||||||||
Timmerman (2006) [20] | 70 | 50 % T1 | 60 Gy (3) | 17.5 | 95 % at 2 years | 100 | 10 | 55 % at 2 years |
50 % T2 | ||||||||
Yoon (2006) [34] | 21 | 62 % T1 | 30–48 Gy (3–4) | 13 | 86 % | 100 | 5 | 51 % at 2 years |
38 % T2 | ||||||||
257 | Multi-institutional review | 18–75 Gy (1–22) | 38 | 86 % | 89 | 20 | 47 % at 5 years | |
63 % T1 | ||||||||
37 % T2 | ||||||||
Hof (2007) [32] | 42 | 40 % T1 | 19–30 Gy (1) | 15 | 95 % at 1 year; 68 % at 2 years | 95 | 31 | 65 % at 2 years |
50 % T2 | ||||||||
10 % T2 | ||||||||
Fakiris (2009) [35] | 70 | 49 % T1 | 60–66 Gy (3) | 50 | 88 % at 3 years | 91 | 13 | 43 % at 3 years |
51 % T2 | ||||||||
Baumann (2009) [36] | 57 | 70 % T1 | 45 Gy (3) | 35 | 93 % | 95 | 16 | 60 % |
30 % T2 | ||||||||
Timmerman (2010) [29] | 55 | 80 % T1 | 60 Gy (3) | 34 | 91 % | 87 | 22 | 48 % at 3 years |
20 % T2 | ||||||||
Ricardi (2010) [37] | 62 | 69 % T1 | 45 Gy (3) | 28 | 88 % at 3 years | 94 | 24 | 57 % at 3 years |
31 % T2 | ||||||||
Bral (2011) [28] | 40 | 65 % T1 | Central: 45 Gy (3) | 16 | 92 % | 95 | 15 | 52 % at 2 years |
35 % T2 | Peripheral: 60 Gy (3) |
Table 13.2
Selected results of lung metastases treated with SABR
Reference (year published) | No. patients (no. lesions) | Patient population | Total dose (no. fractions) | Median follow-up (months) | Local control | Overall survival |
|---|---|---|---|---|---|---|
Wulf (2004) [30] | 41 (51) | Primary site: | 30–37.5 Gy (3) or 26 Gy (1) | 9 | 90 % | 33 % at 2 years |
45 % lung | ||||||
10 % breast | ||||||
8 % colorectal | ||||||
8 % kidney | ||||||
8 % sarcoma | ||||||
Yoon (2006) [34]
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|