© Springer-Verlag Italia 2015
Mirko D’Onofrio, Paola Capelli and Paolo Pederzoli (eds.)Imaging and Pathology of Pancreatic Neoplasms10.1007/978-88-470-5678-7_10Imaging Stigmata Review and Interpretation
(1)
Department of Radiology, San Matteo University Hospital, Viale Camillo Golgi, 19, Pavia, 27100, Italy
Introduction
Pancreatic pathologies are often hard to diagnose, need a primary cooperation of clinicians, radiologists, and pathologists. The imaging diagnosis covers the most important role, being the central node of this teamwork. The evaluation should be directed first toward differentiation between solid and cystic lesions, together with discrimination between benign and malignant behavior, second toward as accurate as possible characterization of the mass. However, both detection and characterization of pancreatic lesions are often not immediate, requiring very often the intervention of more than one noninvasive imaging modalities (ultrasound, computer tomography, and magnetic resonance imaging) and very often the use of invasive procedures for final diagnosis confirmation. As well known in the literature, different pancreatic lesions have some more or less specific imaging features able to help us for the diagnosis. However, each imaging modality offers specific advantages and disadvantages compare to the others and covers a different step in the diagnostic workup of pancreatic pathologies [1]. According to the guidelines, for noninvasive diagnosis, the same result in almost two different diagnostic examinations leads to make the diagnosis, but for pancreatic malignancies a pathologist confirmation is always required before palliative therapies.
Ultrasound
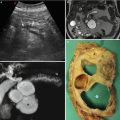
In European and Asian countries, ultrasonography (US) is usually the first diagnostic step in case of suspicion of abdominal pathology, being relatively simple and without any contraindications or side effects. As known, it has higher capability in detecting lesions located in the pancreatic head and body than in the tail of the gland, being the left pancreas more difficult to visualize. Moreover, its accuracy in distinguishing solid and cystic lesions is greatly implemented by the i.v. administration of contrast agent that greatly implements the US ability in characterizing both solid and cystic lesions [2, 3]. CEUS has a diagnostic accuracy in distinguishing solid from cystic lesions of about 90–100 % and a confidence in discerning benign from malignant masses characterized by a ROC curve area of 0.877 [4].
About solid lesions, US shows a diagnostic sensitivity of 90 % and a specificity of more than 90 % in detecting pancreatic adenocarcinoma [5–8]. The accuracy of US in detecting pancreatic adenocarcinoma can be higher than 90 %, especially in the setting of small pancreatic masses; in a study comparing US and Computer Tomography (CT) in 64 patients with adenocarcinoma, US correctly identified 61 (95.3 %) of 64 tumors, whereas CT correctly identified 57 (89.1 %) of the 64 tumors [7].
Concerning neuroendocrine tumors (NETs), US can detect only 60 % of islet cell tumors. In particular, it shows a sensitivity of 24.3 % and an accuracy of 24.3 % in detecting insulinoma, the most frequent type of pancreatic NETs [9].
However, CEUS shows sensitivity, specificity, PPV, NPV, and accuracy of 87.5, 88.3, 94.2, 76.4, and 87.8 %, respectively, in correctly characterizing adenocarcinoma and sensitivity, specificity, PPV, NPV, and accuracy of 73.7, 93.1, 61.9, 95.9, and 90.5 %, respectively, in correctly characterizing neuroendocrine tumors [4].
Concerning the enhancement pattern, a large cohort multicenter study showed that a hypovascular lesion detected at CEUS reflects an adenocarcinoma in 95–96 % of cases, whereas an hypervascular lesion reflects a neuroendocrine tumor in 46.4–62 % [4, 10, 11]. In fact, pancreatic adenocarcinoma typically presents as a hypoenhancing mass at CEUS in about 90 % of the cases [4, 12, 13].
About cystic lesions, CEUS has a sensitivity, specificity, PPV, NPV, and accuracy of 79.4–94 %, 76.6–99.4 %, 66.7–90.4 %, 94.2–98.6 %, and 84.2–98.1 %, respectively, in the differential diagnosis between benign and malignant behavior [14, 15]. In fact, it may significantly improve the diagnostic accuracy of US in distinguishing pseudocyst from cystic tumors, having a higher spatial and temporal resolution, that lead to identify also small enhanced inclusions [16]. At CEUS, typical enhancement pattern of pseudocystic lesion is the avascular aspect in all phases, whereas the presence of intracystic vegetations vascularized excludes the hypothesis of pseudocyst leading to the diagnosis of cystic pancreatic tumor. Its ability in the identification of intracystic nodules is characterized by a sensitivity, specificity, PPV, NPV, and accuracy of 75 % (up to 88.2 % in IPMN as reported by Kurihara) [17], 96, 85.7, 92.3, and 90.9 %, respectively, whereas in the identification of septa of 93.3, 88.8, 87.5, 94.1, and 90.9 %. respectively [12, 18].
Computed Tomography
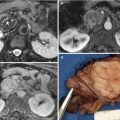
In European countries, computed tomography (CT) is usually the further diagnostic step in case of previous diagnosis of pancreatic solid lesion [1]. The i.v. administration of contrast material is always necessary to permit an accurate diagnosis. The most important contraindication is represented by ionizing radiations, while the more frequent side effects are related to allergic reaction to contrast agent. Obviously, no difference exists in diagnosis of pathologies located in the right or left portion of the gland, but as known, the capability of CT in distinguishing solid and cystic lesions cannot be obvious. CT can overcome the limitation of US in case of obesity, fatty infiltration of the pancreas, and in identifying calcifications. Calcifications are simply detected and their location and morphology may sometimes give further information for the diagnosis.
CT can be considered the most robust examination for the study of solid pancreatic tumors. The sensitivity of CT in detecting pancreatic cancers lies between 75 and 100 % with a specificity of 70–100 % [19]. For lesions more than 2 cm in size, sensitivity can reach 98 % [20, 21]. For lesions less than 2 cm in size, however, the sensitivity is between 68 and 77 % with an accuracy of 77 % [21–24]. In this setting, the presence of secondary signs (such as pancreatic duct cut-off, dilatation of the pancreatic duct or common bile duct, upstream atrophy of the parenchyma, and contour abnormalities), could help in diagnosis of pancreatic cancer [19, 25, 26]. A recent study on 163 patients with small pancreatic adenocarcinomas showed that the prevalence of pancreatic cancer accompanied by secondary signs differs significantly according to tumor size: 76 % of tumors smaller than 20 mm and 99 % of tumors between 21 and 30 mm [27]. The prevalence of isoattenuating pancreatic cancers accompanied by secondary signs was 88 % (14 of 16) for tumors 20 mm or smaller and 100 % (12 of 12) for tumors 21–30 mm [27, 28].
On the other hand, CT is essential for the panoramic evaluation of the disease, necessary in case of diagnosis of pancreatic cancer. The total-body evaluation can be done only by MDCT, to define resectability or unresectability of the lesion and eventually presence of metastatic lesions [29]. MDCT offers a panoramic and complete preoperative staging [1, 30]. MDCT had a sensitivity, specificity, NPV, PPV, and accuracy of 79–94 %, 82–84.2 %, 91–94 %, 62–84 %, and 81–91.3 %, respectively, in predicting unresectability of adenocarcinoma [31], with a sensitivity and specificity of 77 and 81 % in the diagnosis of vascular invasion [32].
In 90–95 % of the cases, adenocarcinoma is a hypoattenuating mass at CT [33], being isoattenuating in 5.4 % of cases [24, 28, 34, 35], that however are 27 % of small (<2 cm in size) lesions.
In a recent study on 30 patients with isoattenuating adenocarcinoma, in all cases at least one abnormal finding in the pancreaticobiliary system has been documented on CT: 26/30 patients (86.7 %) showed abrupt cut-off of pancreatic duct with upstream dilatation, 24/30 (80 %) showed narrowing of the distal common bile duct and upstream dilatation, whereas only 4 of 30 (13.3 %) showed abrupt cut-off of pancreatic duct and upstream pancreatic atrophy, and 4/30 (13.3 %) abrupt cut-off of pancreatic duct and mild infiltration around upstream pancreas. A focal pancreatic contour bulging at tumor location was seen in 3 out of 30 cases (10 %), a small intrapancreatic retention cyst adjacent to the lesion in 3/30 (10 %), whereas in 1 out of 30 (3.3 %) cases soft-tissue cuffing around celiac trunk was noticed at CT [34].
MDCT shows a detection rate of 69–94 % in identifying neuroendocrine tumors, typically showing as a hypervascular mass in up to 90 % of cases, showing cystic features in 17 %, calcifications up to 20 % in nonfunctioning NETs [36].
In a study on 60 patients with NETs, MDCT has proved to be useful in predicting uncertain behavior or malignancy, based on pattern and tumor mean size. The PPV of malignancy according to tumor size varies from 44 % (lesion smaller than 2 cm), 57 % (lesions from 2 to 3 cm), and 61 % (lesions more than 3 cm). It has been shown that a solid pattern is predictive of malignancy respectively in 50, 68, and 89 % according to the lesions size (<2 cm, 2–3 cm, and >3 cm). Calcifications could be observed respectively in 0, 50, and 100 % (lesions <2 cm, lesions 2–3 cm, and lesions >3 cm). A cystic pattern, a typical uniform peripheral rim of enhancement without septations or mural nodules was observed in 0, 45, and 43 %, respectively (lesions <2 cm, lesions 2–3 cm, and lesions >3 cm). A complex appearance, with an enhancing thick irregular wall, thin irregular septa, or mural nodules was shown respectively in 0, 54 and 45 % (lesions <2 cm, lesions 2–3 cm, and lesions >3 cm) [37].
Magnetic Resonance
On the other hand, in European and Asian countries, magnetic resonance imaging (MRI) is usually the further diagnostic step in case of previous diagnosis of pancreatic cystic lesion owing to its highest capability in detecting fluid contents [38]. The MRI study of the pancreatic ductal system with cholangiopancreatography (MRCP) has to be always performed to accurately evaluate the relationship between lesion and ductal system [39, 40]. The i.v. administration of contrast material is always necessary to allow an accurate diagnosis. The most important contraindications are represented by the well-known limits of magnetic fields, whereas allergic reactions to contrast agent are rare. Obviously, also at MRI no difference exists in diagnosis of pathologies located in the right or left portion of the gland, but the time examination is significantly longer than that of CT and so, this imaging modality should be reserved for cystic lesions [1]. As reported for CT, MRI can overcome the limitation of US in case of obesity and fatty infiltration of the pancreas.
The detection of a pancreatic cystic lesion has to be followed by the identification of a potential communication with the ductal system to allow the differentiation between IPMN and non-IPMN pathologies [41–45]. The sensitivity, specificity, and accuracy of MRI with MRCP are of 91.4–100 %, 89.7 %, and 90.5 %, respectively [46–49]. As well reported in the literature, MDCT has a high, but slightly lower in respect to MRI with MRCP, (sensitivity of 83–87.5 % and accuracy of 77.4–79 %) in the assessing of communication with the main pancreatic duct, thanks to curved MPR post-processing examinations [46, 49–51].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree