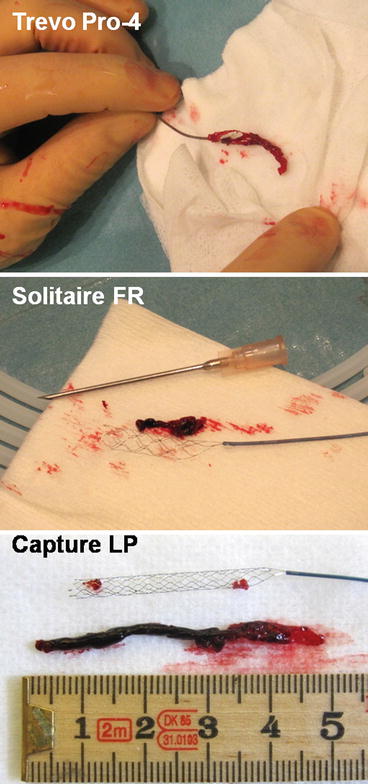
Fig. 1
Example of a flow chart used at the Karolinska University Hospital
The neurointerventional stroke center should be operational 24/7, the neurointerventionalists and stroke neurologists easily contacted through special phone numbers, preferably having access to telemedicine.
Referring hospitals should, if possible, be trained to perform CT and CT angiography (CTA) before contacting the stroke center. Patients eligible for IV thrombolysis transferred from referring hospitals may receive the infusion in full concentration (0.9 mg/kg rt-PA with a maximum dose of 90 mg) as bridging therapy and transported acutely with the drip ongoing. It is important that the IV infusion do not delay the transportation to the neurointerventional stroke center, instead it can be administered while the transfer is being organized. It is almost always quite sufficient to have well-trained paramedics looking after the patient during the transportation.
The transferred patients should if possible bypass the emergency room (ER) and instead come straight to the neurointerventional center for further evaluation and potential mechanical thrombectomy. When the patient arrives, representatives (physicians, nurses, and technicians/technologists) for all the involved medical specialities should be present: stroke neurology, diagnostic and interventional neuroradiology as well as anesthesiology.
Patients that are brought directly to the emergency department of a hospital that perform neurointerventional therapy should have the CT performed at the earliest possible time point, and, if eligible, receive IV treatment immediately after the scan at the neuroradiology department and not be transported elsewhere for the infusion. The time at the ER should ideally not exceed 10 min before the CT is performed; brief medical history is noted, respiration and blood pressure (BP) are investigated, NIHSS is calculated, and potential contraindications for IV treatment are determined. There is no need for blood samples at this moment.
If possible, also these patients not transferred from other hospitals should instead be brought straight to the neurointerventional center, preferably after telephone contact between the paramedics and the stroke neurologist on call in an attempt to rule out as many stroke mimics as possible. Such patients with other diagnoses have to be transported secondarily to the ER.
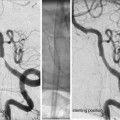
Medical management before thrombectomy may follow standard guidelines as published by the European Stroke Organisation or the American Stroke Association. It is important not to lower a high BP too extensively, accepting a systolic pressure ≤200, as this pressure may be necessary to perfuse the brain parenchyma distal to the clot through so-called pial collaterals (Fig. 2). Such collaterals, which emerge from other vascular territories, vary significantly between different individuals and are the structural basis behind the survival of threatened brain tissue. Without pial collaterals, the area supplied by the occluded artery would survive no more than perhaps 10–15 min. This is also reflected in the fact that it is very difficult to avoid basal ganglia infarction in an MCA main stem occlusion. This central area is mainly supplied by end arteries, i.e., the lenticulostriate perforators, in such case obstructed by an MCA (M1) thromboemboli. As there is limited collateral supply to the basal ganglia, an ischemic infarct will consequently develop rapidly.
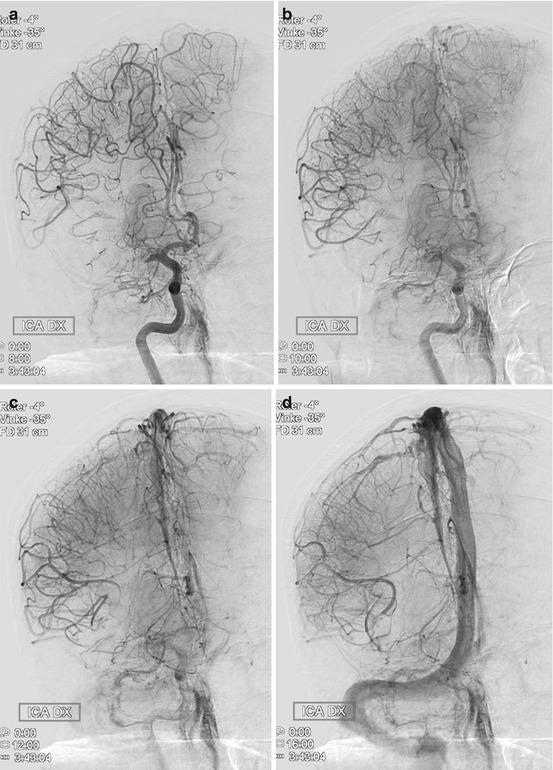

Fig. 2
Thromboembolic M1 occlusion on the right side with prominent pial collaterals retrogradely supplying the MCA territory. (a) Late arterial phase (b) Early capillary phase (c) Late capillary phase, (d) Early venous phase. The patient was successfully treated with mechanical thrombectomy resulting in a NIHSS decrease from 19 to 4 in 24 h
Patient Selection
Patient selection is the key to good outcome for the treated patients as well as to avoid complications.
There is no absolute time limit from symptom onset before which mechanical thrombectomy has to be started. Instead, patient selection should be based on clinical and radiological criteria. In practice, it is however rare that a patient may benefit from thrombectomy if the procedure starts >8 h after onset in the anterior circulation and >24 h after onset in the posterior circulation.
Clinical
Anterior circulation: NIHSS 6–25. If the patient has a score <6, the risk/benefit ratio becomes unfavorable. A lower score may be accepted if the patient has aphasia as this may be viewed as a devastating handicap. If the patient has a score >25, he/she is usually in a too bad condition to be helped by a thrombectomy procedure.
Posterior circulation: NIHSS is not readily applicable. The selection is especially difficult as even patients in Glasgow Coma Scale (GCS) 3–4 may have a good outcome. Clinical fluctuations are usually a good sign.
There is no absolute age limit for thrombectomy, but high biological age should be a restricting factor as well as many significant comorbidities. These may impose anesthesiological problems (e.g., cardiopulmonary restrictions), affect the possibility for recovery and rehabilitation (e.g., contralateral stroke, dementia), and long-time survival (e.g., cancer with bad prognosis).
Radiological
Anterior Circulation
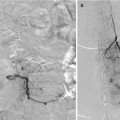
CT-based radiological protocols for selection of patients with anterior circulation stroke can be recommended to contain a non-contrast enhanced CT, CTA, and CT perfusion (CTP, Fig. 3). The CTA provides invaluable information about the status of the arteries from the aortic arch to the intracerebral circulation. Not only can the exact location of the thromboemboli be determined but obstacles on the way (e.g., carotid stenosis) are discovered making it possible to plan the procedure beforehand. The basal axial images called CTA-SI (source images) can be used not only to estimate the extent of pial collaterals but also to more clearly delineate the infarcted tissue by adjusting the window level and width. As CTP still may be regarded uncertain, for one thing because of difficulties to determine thresholds, the status of the ischemic brain tissue can consequently be supplementary estimated on the basis of CT and CTA, preferably utilizing the so-called ASPECTS score, possible on both non-contrast CT and CTA-SI.
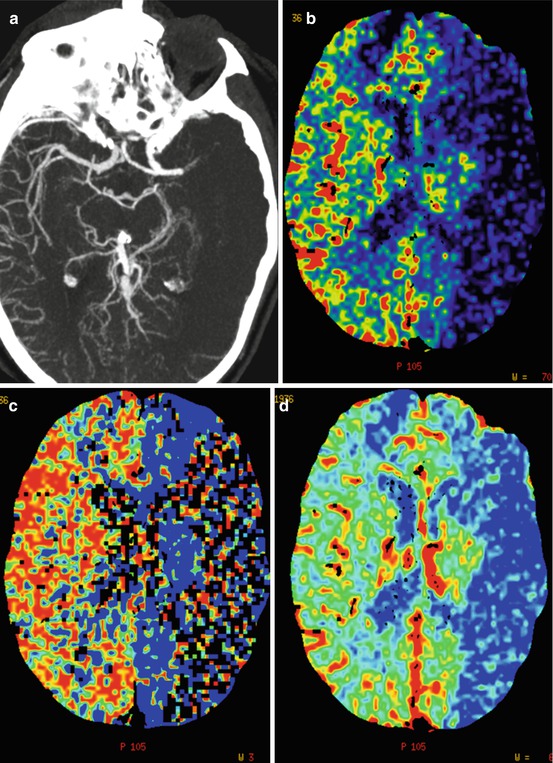
Fig. 3
CTA and CTP from a patient scanned 1.5 h after symptom onset. (a) CTA (b) CTP – blood flow (c) CTP – mean transit time (d) CTP – cerebral blood volume. Note the left-sided MCA occlusion, lack of pial collaterals, and markedly decreased blood flow and cerebral blood volume in large areas of the MCA-territory. The patient also deteriorated clinically very rapidly and it was decided not to pursue a thrombectomy procedure
Alternatively, the protocol may be based on MR and should then, in addition to a CT which is usually always done, consist of at least time-of-flight (TOF), diffusion weighted images (DWI), and T2-weighted images/Flair. MR perfusion is usually not necessary as the focus should be on detecting brain parenchyma already infarcted. This means that blood volume in CTP and DWI in MR become especially important. In large vessel occlusions, no extensive infarct present usually means salvageable tissue. Under such circumstances, there are always threatened areas, so-called penumbra, kept alive by pial collaterals. In contrast, large infarcts (>1/3–1/2 of the MCA territory, so-called malignant MCA-pattern) imply bad patient outcome even with successful revascularization and a thrombectomy procedure in that context only adds risk for reperfusion hematomas/hemorrhagic conversion in addition to the risk for complications from the procedure itself.
Posterior Circulation
For radiological selection of patients with posterior circulation stroke, usually BAO, it may be recommended to include MR-sequences in the selection process. The protocol can consist of CT, CTA (alternatively MR-TOF), CTA-SI, MR-DWI, and MR-T2WI/Flair. It is crucial to detect brain stem infarcts, especially mesencephalic infarcts, as they are strong predictors for bad outcome. The presence of significant mesencephalic infarcts speaks against thrombectomy as it probably is too late for saving or helping the patient by revascularization.
Treatment Protocol
If possible, it is preferred to perform the thrombectomy under conscious sedation (CS) instead of general anesthesia (GA) for several reasons. CS is faster and it is possible to have better control of the patient. Most importantly though, the patients always get hypotensive at induction of GA unless the BP drop is counteracted with vasopressors (e.g., noradrenaline/norepinephrine), something that is rarely done for acute stroke patients. As these patients rely on pial collaterals, they need a high-cerebral perfusion pressure and a BP decrease may be deleterious, pushing ischemic/oligemic tissue into manifest infarct. If GA is inevitable, special care should always be taken to meticulously avoid blood pressure drop.
There is no need for anticoagulation or antiaggregation during a thrombectomy procedure other than a standard dose of heparin in the flushes (e.g., 5 IU/ml) unless the patient is permanently stented. This should, however, be avoided as the necessary medication to keep the stent patent may increase the risk for reperfusion hematomas/hemorrhagic conversion. If it is necessary to leave a stent, Gp IIb/IIIa inhibitors can be used in the acute situation. For example, half bolus of abciximab can be administered IV when the stent is in place followed by a loading dose of clopidogrel (300 mg) and acetylsalicylic acid (ASA) (300–500 mg) after 12–24 h, usually in the next morning. If the patient is resistant to clopidogrel, the dose may be doubled or changed to prasugrel (bolus 60 mg). The patient should be kept on double medication, clopidogrel 75 mg (alternatively prasugrel 10 mg)/ASA 75–325 mg daily for at least 3 months and on ASA alone for another at least 3 months. Alternatively, if the patient is regarded be at a specifically high risk for intracerebral hemorrhage, a loading dose of ASA (300–500 mg) alone may be administered orally, IV, or per rectum (PR) immediately after stent placement. Clopidogrel (or prasugrel) is than added if a 22–36 h post-procedure CT is negative, i.e., with no symptomatic intracerebral hemorrhage according to the so-called ECASS definition. This latter pharmacological regime may, however, pose a slightly higher risk of immediate or delayed stent thrombosis.
Basic Procedural Protocol for Thrombectomy with a “Stent Retriever” in the Anterior Circulation
There are several stent retrievers available today with different advantages and disadvantages. This protocol is applicable to all of them (Fig. 4).