The sensitivity of mammography is more limited in patients with dense breasts and some patients at higher risk for breast cancer. Patients with intermediate or high risk for breast cancer may begin screening earlier and benefit from supplemental screening techniques beyond standard 2-dimensional mammography. A patient’s individual risk factors for developing breast cancer, their breast density, and the evidence supporting specific modalities for a given clinical scenario help to determine the need for supplemental screening and the modality chosen. Additional factors include the availability of supplemental screening techniques at an individual institution, cost, insurance coverage, and state-specific breast density legislation.
Key points
- •
Patients with an intermediate or high lifetime risk for breast cancer may benefit from supplemental screening beyond 2-dimensional mammography to improve the detection of breast cancer.
- •
Supplemental screening modalities beyond 2-dimensional mammography include digital breast tomosynthesis, contrast-enhanced spectral mammography, ultrasound examination, MR imaging, and molecular breast imaging.
- •
The decision to perform supplemental screening depends on individual risk status, evidence to support a specific modality for a given clinical scenario, cost, and availability.
- •
Prospective, multi-institutional trials of supplemental screening with direct comparison of the recall rate, cancer detection rate, and false-positive examinations are needed.
Introduction
The early detection of breast cancer with screening mammography in women who undergo screening has been well-established in multiple trials to decrease mortality by more than 40%. However, the sensitivity of mammography is more limited in patients with dense breasts and some patients at higher risk of breast cancer (as low as 19%–37%). Patients with an intermediate or high lifetime risk for breast cancer may begin screening at an earlier age and benefit from supplemental screening beyond standard 2-dimensional (2D) mammography to improve the early detection of breast cancer. The determination of whether or not supplemental screening is implemented for a given patient, and the modality chosen, is based on several factors. These parameters include the patient’s individual risk factors for developing breast cancer (personal or family history of breast cancer, genetic mutation, history of atypia or lobular hyperplasia, nulliparity), imaging data such as mammographic breast density, and the evidence supporting a specific modality for supplemental screening given the clinical scenario. Additional factors for consideration include the availability of supplemental screening techniques at an individual institution, cost, insurance coverage, patient and physician preferences, and state-specific breast density legislation.
This article includes a review of risk profiles that may be considered for supplemental screening, breast density, types of supplemental screening modalities, and current national guidelines.
Discussion
High-Risk Screening
Genetic predisposition
Hereditary breast and ovarian cancer syndrome reflects the presence of a genetic mutation that confers a higher risk for the development of breast and ovarian cancers. Inherited genetic mutations are implicated in approximately 5% to 10% of breast cancers, with the most common genetic mutations affecting the BRCA1 or BRCA2 genes. The estimated lifetime risk for the development of breast cancer is up to 87% for a BRCA1 gene mutation carrier and 56% for a BRCA2 gene mutation carrier. In patients with a known pathogenic (or likely pathogenic) BRCA gene mutation, screening is recommended to begin by age 25 ( Table 1 ). ,
| Patient Group | ACR | NCCN |
|---|---|---|
| BRCA , | MR imaging ≥25–30 y | MR imaging ≥25–29 y to 75 y a |
| MG ≥30 y b | MG ≥30–75 y | |
| Li–Fraumeni syndrome (TP53) , | MR imaging ≥25–30 y | MR imaging ≥20–29 y to 75 y |
| MG ≥30 y | MG ≥30–75 y | |
| >75: Individual management | ||
| Cowden syndrome/PTEN hamartoma tumor syndrome , | MR imaging ≥25–30 y | MG + MR imaging 30–35 y c to 75 y |
| MG ≥30 y | ||
| Peutz-Jeghers syndrome (STK11) , | MR imaging ≥25–30 y | MG + MR imaging ≥25y |
| MG ≥30 y | ||
| CHEK2/ATM/NBN with 657deI5 variant , | MR imaging ≥25–30 y | MG + consider MR imaging ≥40 y |
| MG ≥30 y | ||
| PALB2/CDH1 , | MR imaging ≥25–30 y | MG + MR imaging ≥30y |
| MG ≥30 y | ||
| NF1 | Not specifically discussed | MG ≥30 y |
| Consider MR imaging 30–50y | ||
| Untested first-degree relatives of mutation carriers , | MR imaging ≥25–30 y | Recommend |
| MG ≥30 y | ||
| ≥20% lifetime risk owing to family history , | MG ≥30 y | MG + MR imaging 10y before youngest family member |
| MR imaging ≥25–30 y | MR imaging not <25 y d | |
| MG not <30 y d | ||
| Chest radiation , | MG + MR imaging age 25 or 8 y after treatment d | MG + MR imaging 10 y after treatment |
| No MG <30 y d | ||
| LCIS or ADH/ALH , | MG begin at diagnosis | MG begin at diagnosis |
| Consider MR imaging | Consider MR imaging | |
| No MG <30 y e | ||
| No MR imaging <25 y e | ||
| Dense breasts (alone) , | MG ≥40 y | MG ≥40 y MR imaging: Insufficient evidence US: Can increase CDR, but may increase recall/benign biopsies MBI: Not recommended |
| Consider US ≥40 y MR imaging: Insufficient data MBI: not recommended | ||
| Personal history of treated breast cancer , | MG begin at diagnosis | Consider MR imaging if lifetime risk of second primary cancer >20% |
| MR imaging recommended if dense breasts or diagnosis <50 y e | ||
a Individualize for >75 y and family history <30 y.
b BRCA1 may consider delaying MG until 40.
c Or 5–10 y before the earliest breast cancer, whichever first.
Although BRCA1 and BRCA2 are the most well-known gene mutations, pathogenic variants in multiple other cancer predisposition genes may also increase risk for breast cancer. Evidence characterizing breast cancer risk for certain gene mutations have led to additional recommendations for breast cancer screening in these patients, including TP53 (Li–Fraumeni syndrome), CDH1 (hereditary gastric cancer syndrome), PALB2, ATM (ataxia–telangiectasia), NBN, CHEK2, STK11 (Peutz–Jeghers syndrome), and Cowden syndrome/PTEN hamartoma tumor syndrome. The age at which to start and the modality of screening varies depending on the pathogenic variant (see Table1 ).
Family history
Patients without a known pathogenic variant in a gene conferring an increased risk for breast cancer, but with a significant family history of breast cancer, remain at higher risk for the development of breast cancer. Once a patient’s lifetime risk reaches 20% or higher, supplemental screening with breast MR imaging in addition to mammography is recommended. , , Individual risk assessment should be based on models that are largely dependent on family history, such as Claus, BRCAPRO, and Tyrer-Cuzick (IBIS). , , Both the number of family members with breast cancer (particularly first-degree relatives) and their age at diagnosis are important considerations in addition to other factors depending on the specific model in use. Screening begins between the ages of 25 and 30, or 10 years before the earliest affected relative (see Table 1 ).
Chest radiation
Women who have received radiation to fields encompassing the chest region that may include the breast tissue, such as thorax, whole lung, mediastinal, axilla, minimantle, subtotal lymphoid, high abdominal, and total body irradiation, are at higher risk for the development of breast cancer. , These women are most often survivors of childhood, adolescent, and young adult cancers such as Hodgkin’s lymphoma and have much improved long-term survival compared with previous decades. However, they are at substantially higher risk for the development of breast cancer, with a cumulative incidence of 13% to 20% by age 45, similar to that of BRCA gene mutation carriers. , Screening begins at age 25 or 8 to 10 years after the completion of radiation, whichever occurs last , (see Table 1 ).
Intermediate-Risk Screening
Personal history of breast cancer
Patients with a personal history of treated breast cancer are at higher risk for the development of a subsequent breast cancer. This second breast cancer could be a second primary tumor in either breast or a recurrence in the breast, chest wall, or axillary or internal mammary lymph nodes. After undergoing breast conservation therapy, the recurrence rate is 0.5% to 1.0% per year and the risk to develop a second cancer is 5% to 10% in the first decade after diagnosis ; and survival is known to improve with the early detection of a second breast cancer. Screening mammography has a lower sensitivity in patients with a personal history of treated breast cancer. Thus, some guidelines support the use of supplemental screening in this patient population, particularly in those patients with dense breasts or a younger age at diagnosis (<50 year old).
High-risk lesions
A personal history of biopsy proven lobular neoplasia (lobular carcinoma in situ or atypical lobular hyperplasia) or atypical ductal hyperplasia (ADH) increases risk for the subsequent development of breast cancer. The increased risk is most significant with lobular neoplasia, which portends a lifetime risk of 10% to 20%. ADH also increases risk, but to a lesser degree than lobular carcinoma in situ. The relative risk for invasive cancer owing to ADH is 4- to 5-fold higher, but it is 6- to 10-fold higher for lobular carcinoma in situ. , Imaging surveillance typically begins at diagnosis (see Table 1 ).
Dense breasts
Mammographic breast density is routinely categorized into 1 of 4 categories by the interpreting radiologist during clinical mammographic interpretation. , Approximately 50% of women are in the 2 highest breast density categories (heterogeneously and extremely dense) ( Fig. 1 ). Compared with women of average breast density (between categories b and c), the relative risk of developing breast cancer if the breasts are heterogeneously dense is less than 1.2 and for extremely dense breasts it is less than 2.1. Dense breasts increase the risk for the development of breast cancer, and patients in the highest density category (extremely dense) have a 4 to 6 times greater likelihood of developing breast cancer than those in the lowest breast density category (fatty), although caution should be taken with this sort of comparison because less than 10% of the population fall into either of those 2 extreme categories. , Dense breasts also have a well-known masking effect on mammography, decreasing the sensitivity for breast cancer detection. Some studies support supplemental screening beyond 2D mammography in patients with dense breasts given the combination of decreased mammographic sensitivity and increased risk for breast cancer.
After legislation at a state-by-state level, federal breast density notification requirements were passed in 2019. These requirements direct the US Food and Drug Administration to implement breast density reporting requirements via the Mammography Quality Standard Act. Once implemented, the federal guidelines will require that mammography reports and summaries received by patients and their providers include information about the effect of breast density in masking the presence of breast cancer on a mammogram, the qualitative density assessment given by the radiologist who interpreted the mammogram, and a reminder to patients that they should talk with their provider if they have dense breasts and have questions or concerns. Some state-specific legislation also mentions supplemental screening and may legislate insurance coverage, although insurance coverage inclusion is less common.
Modalities
Digital breast tomosynthesis
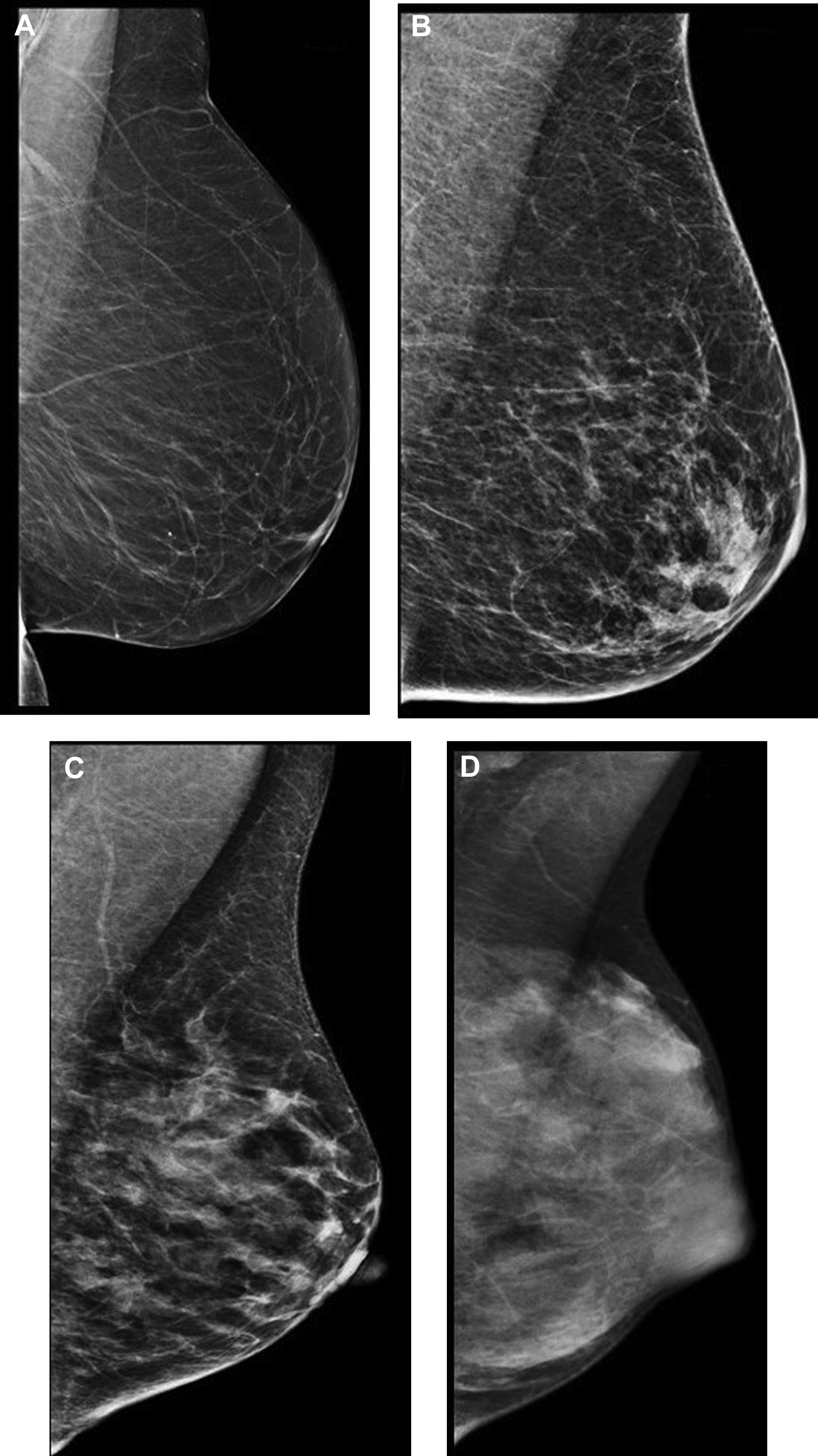
Digital breast tomosynthesis (DBT) is a digital mammogram technique in which tomosynthesis images are constructed from multiple low-dose images acquired as the x-ray source moves in an arc over the breast. Compared with standard digital mammography (DM), DBT decreases the masking effect by decreasing superimposition from overlying breast parenchyma. Both prospective and retrospective studies have demonstrated decreased recall rates and increased cancer detection rates (CDRs) with DBT compared with DM alone ( Fig. 2 ).
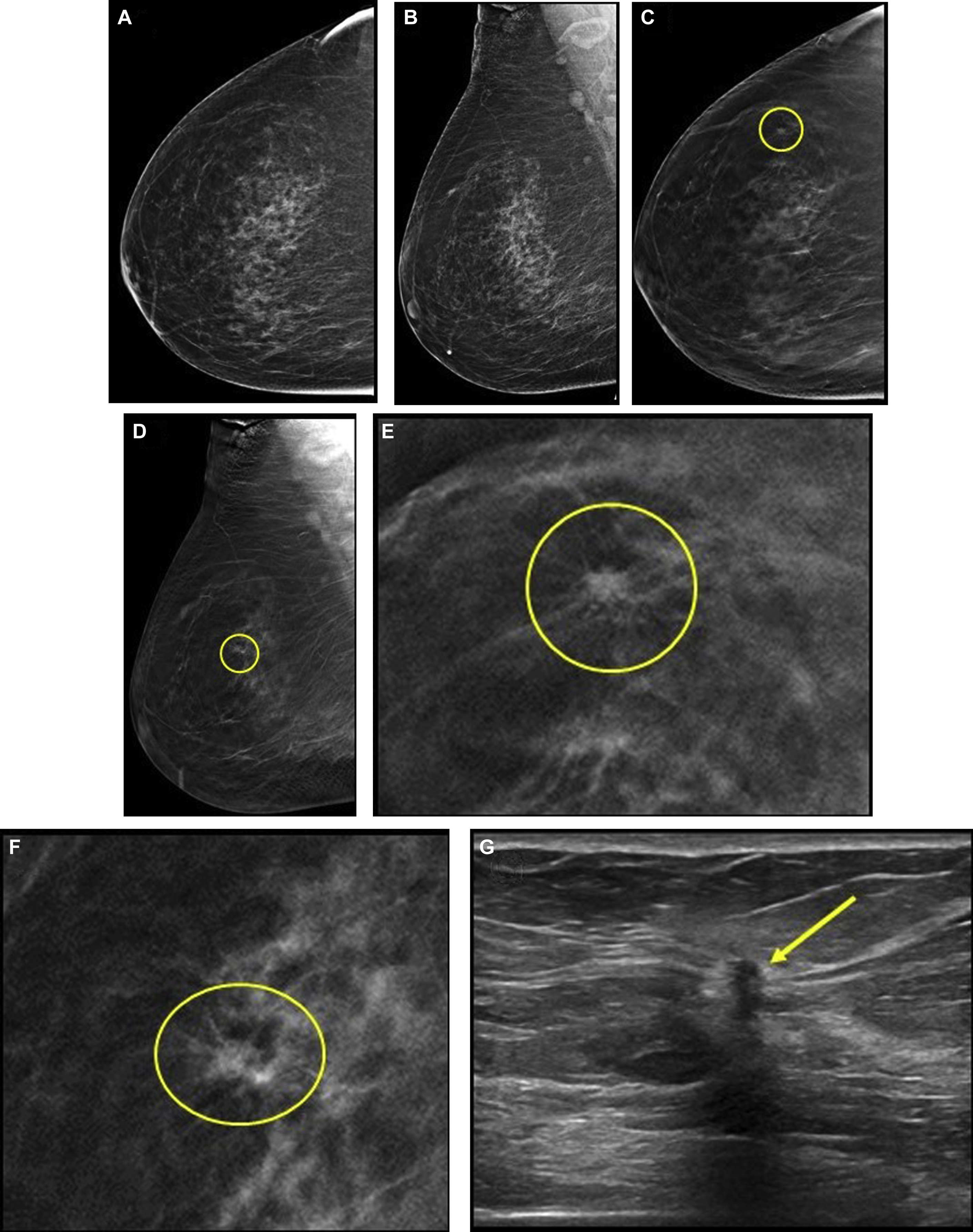
Although screening outcomes for DBT in specific high-risk populations are limited, because most studies did not include patient-level risk factors, the effects of DBT in higher risk women are likely similar to those described in average risk women. A retrospective study including 2673 women at increased risk (owing to personal history or first degree relative with breast cancer) reported a higher CDR for combined DBT and DM versus DM alone in both women at average risk (5.1 vs 4.5 per 1000) and increased risk (8.6 vs 7.9 per 1000); however, this difference was not statistically significant. In a prospective study of 618 women with a personal history of breast cancer, the addition of DBT decreased the recall rate when compared with DM alone. In contrast, in a randomized trial of women in their forties with a family history of breast cancer, the addition of DBT to DM in incident screening did not lead to a significant reduction in recall rate, although the DM recall rate was very low at 2.8%. Data from the Tomosynthesis Mammographic Imaging Screening Trial (TMIST), which will include patient age, cancer risk, and breast density, will hopefully provide more information on screening outcomes for DBT in high-risk populations.
For intermediate-risk women with dense breasts, DBT screening has a pooled incremental CDR (ICDR) beyond standard 2D mammography of 3.9 per 1000 in prospective screening trials and of 1.4 per 1000 in retrospective studies, with a significant decrease in the recall rate, with a pooled difference of −23.3 recalls per 1000 screens. Overall, DBT screening in women with dense breasts is estimated to have an ICDR of 1 to 3 per 1000 examinations, a positive predictive value of biopsy (PPV3) of 25%, and a recall rate of 7% to 11%. ,
Although studies have demonstrated improved CDRs and a decrease in the recall rates with DBT across breast density categories, including in women with dense breasts, cancers can be obscured if there is lack of a visible interface between the lesion and surrounding breast parenchyma. This factor may account for the higher ICDR seen with supplemental screening ultrasound (7.1 per 1000) compared with supplemental DBT (4 per 1000) in women with dense breasts and negative DM in the ASTOUND trial, although at the expense of a significantly increased false-positive rate for ultrasound examinations. Given that DBT detected more than 50% of additional breast cancers compared with DM alone, however, it is likely a superior primary screening modality for women with dense breasts compared with conventional 2D DM, and may be considered for primary screening per National Comprehensive Cancer Network and American College of Radiology guidelines.
Contrast-enhanced spectral mammography
Contrast-enhanced spectral mammography (CESM) is an emerging technique that combines morphologic data from DM with functional data from iodinated contrast uptake secondary to tumor angiogenesis for breast cancer detection.
A meta-analysis of CESM reported a pooled sensitivity of 98% and specificity of 58%. In a study of 72 women with dense breasts who underwent CESM and mammography, CESM had significantly higher accuracy (90.9%), sensitivity (86.2%), and specificity (94.1%) compared with mammography. Although most studies have focused on the diagnostic usefulness of CESM, few studies have investigated its use as a screening tool. A pilot study comparing screening CESM and MR imaging for women at increased risk for breast cancer found that both modalities identified cancers not seen on DM, with a CESM recall rate of 7.5%, a PPV3 of 15.4%, and a specificity of 94.7%. In a study of 611 women at intermediate risk who have dense breasts, screening CESM was significantly more sensitive than DM (90.5% vs 52.4%), with a specificity of 76.1%; a PPV of 11.9%; a negative predictive value of 99.6%; and an ICDR of 13.1 per 1000. Seven of 8 cancers seen only with CESM were invasive (mean size, 9 mm). Results of a larger study of 904 women at increased risk undergoing screening CESM reported improved performance compared with DM, with increase in sensitivity from 50.0% to 87.5%; a specificity of 93.7%; a PPV3 of 29.4%; an ICDR of 6.6 per 1000; and a BI-RADS category 3 rate of 2.8%.
Whole breast ultrasound examination
Ultrasound examination has several unique benefits as a supplemental screening modality: it is widely available, relatively inexpensive, well-tolerated by patients, and does not involve intravenous contrast administration (as with MR imaging) or radiation (as with molecular breast imaging [MBI]). Screening breast ultrasound examinations may be performed via handheld technique (technologist or physician) or automated scanner, and interpreted in either real time or batch fashion akin to screening mammography.
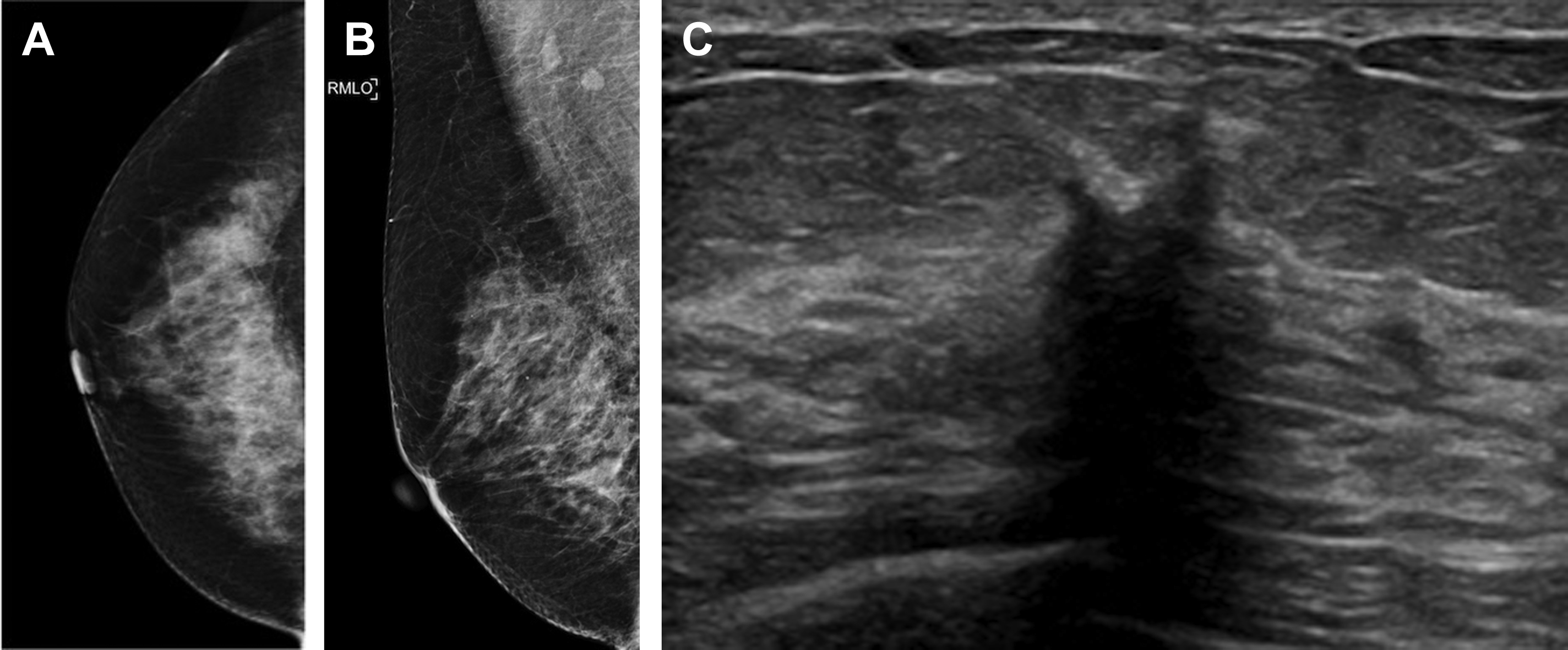
Multiple studies have demonstrated the usefulness of screening breast ultrasound imaging in identifying mammographically occult cancers, with an ICDR of 1.8 to 4.6 per 1000 women screened ( Fig. 3 ). Of incremental cancers identified via screening breast ultrasound, the majority are small (mean size, 10 mm), invasive, node-negative cancers. By detecting cancers masked by breast parenchyma on mammography, supplemental screening ultrasound examination decreased the interval cancer rate by 50% in the Japan Strategic Anti-Cancer Randomized Trial (J-START). A study of women with breast cancers detected at screening ultrasound examination reported excellent outcomes, with a 5-year overall survival rate of 100% and recurrence-free survival rate of 98%.
In high-risk women undergoing both screening mammography and MR imaging, ultrasound examination does not yield any additional cancer detection and should be reserved for further evaluation or biopsy of abnormalities identified on mammography or MR imaging. , For women with an increased risk who qualify for but cannot undergo MR imaging (for reasons including implants or devices incompatible with MR imaging, severe claustrophobia, and body habitus), supplemental screening with ultrasound examination should be considered. The American College of Radiology Imaging Network 6666 trial was a multicenter prospective trial evaluating the performance of physician handheld screening ultrasound examinations in women with an increased risk and dense breast parenchyma. The ICDR for ultrasound examination was 5.3 per 1000 during the prevalence round and 3.7 per 1000 during the 2 incidence rounds, for an average ICDR of 4.3 per 1000. , This increased cancer detection, however, was offset by increased false positives, with a false-positive rate of ultrasound examination alone of 8.1% (vs 4.4% for mammography). The PPV3 for ultrasound-only findings was 9% for prevalence screening and 11.7% for incidence screening (vs 38.1% for mammography). Although performance improved with increased experience and comparison examinations, there was still a substantial rate of biopsy secondary to ultrasound screening, averaging 5% of all participants. After 3 rounds of screening with mammography and ultrasound examination, a subset of patients also underwent screening MR imaging, with an ICDR of 14.7 per 1000, demonstrating that ultrasound examination may still miss many cancers detected by MR imaging.
After the implementation of breast density legislation in Connecticut, data for technician-performed handheld ultrasound in women in the general population with dense breast parenchyma was published, with ICDR of 3.2 per 1000 and a PPV3 of 6.5% to 6.7%, lower than values reported for patients at elevated risk. , In women with dense breasts undergoing screening ultrasound examination, a retrospective study found no significant difference in additional cancer detection after DM versus after DBT.
Factors limiting the implementation of screening breast ultrasound examinations include operator dependence, , a high-false positive rate (with recall rates twice as high and biopsy rates 2–3 times as high as screening mammography), a low PPV of biopsy recommendations, , and a high rate of BI-RADS 3 lesions requiring short-interval follow-up, ranging from 9% to 20%, The technique is also time and labor intensive, with mean performance times ranging from 4 to 19 minutes for physician handheld ultrasound examination , and 10 minutes for technologist handheld ultrasound examination.
Owing to the known limitations of handheld ultrasound examination, automated breast ultrasound (ABUS) examination systems have been developed to eliminate physician time for image acquisition and minimize operator dependence ( Fig. 4 ). Studies have shown improved performance with the combination of mammography and ABUS examination. Kelly and colleagues showed an ICDR of 3.6 per 1000 screening examinations in women with dense breasts at increased risk for breast cancer undergoing ABUS examination. In a multi-institutional study evaluating more than 15,000 asymptomatic women with dense breasts, ABUS examination detected an additional 1.9 cancers per 1000 women. Another study of ABUS examination in women with dense breasts reported an ICDR of 2.4 per 1000 with 9 additional recalls per 1000 women screened.