, Francesca Abbatini1 and Gianfranco Silecchia1
(1)
General and Bariatric Surgery Unit, Medico-Surgical Sciences and Biotechnologies-Sapienza University of Rome, Latina, Italy
Laparoscopic adjustable silicone gastric banding (LAGB ) was the first bariatric procedure to be performed by a laparoscopic approach. Introduction of LAGB into clinical practice was an immediate success in Europe as well as in Australia. Although sleeve gastrectomy, standard Roux-en-Y gastric bypass (RYGBP ), and biliopancreatic diversion with duodenal switch (BPD-DS ) currently represent the majority of laparoscopic bariatric/metabolic procedures in the United States and Canada, laparoscopic gastric banding during the last 10 years has been growing acceptance by physicians as well as by patients. The idea behind the operation is to “create” a small pouch in the upper part of the stomach with a controlled and adjustable stoma, without stapling, thus limiting the daily food intake (restrictive procedure). The silicone prosthesis is placed around the stomach just below the gastroesophageal junction, creating a 15–20 mL pouch (virtual pouch) (Fig. 2.1). This operation does not involve neither rerouting of food through the upper gastrointestinal tract nor exclusion of intestinal segments. The weight loss process in the short and long term is due to the food intake restriction and early satiety. In the highest quality study, excess body weight loss at 1 year after LAGB is 48%. At this time the hypertension, diabetes, dyslipidemia, and sleep apnea resolution rate were about 55%, 58%, 42%, and 45%, respectively [1]. The LAGB represents the bariatric procedure with the lower reported incidence of short- and midterm adverse events [2–4]; however, long-term data show a higher incidence of postoperative acute complications leading to band repositioning or removal and eventually conversion to other procedures [5–7].


Fig. 2.1 Gastric banding
2.1 Pouch Dilation
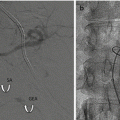
The major complications are pouch dilation (acute or chronic) often referred to as “slippage ,” erosion, and permanent or recurrent outlet obstruction. Slippage is the most common LAGB complication and the leading cause of reoperation. It can develop early or late during the postoperative course. It reported incidence of 1–20%, in the published series [5–12], dropped (event 0.9%) after the surgical technique, and the prosthesis evolved during the years [5, 9, 13]. It consists in the dilatation of the gastric pouch, above the band, in three different modalities: anterior, posterior, or symmetrical. Chronic pouch dilation presents with a gradual onset of symptoms as food intolerance, dysphagia, decrease in satiety, and sense of restriction. Its diagnosis and treatment are usually managed by the bariatric surgeon. An acute slippage is characterized by persistent abdominal pain, vomiting, and eventually obstructive symptoms. The incidence of acute slippage dropped to about 2–10% after the positioning technique of the band through the pars flaccida has been generally adopted (24% with the initial peri-gastric technique) [5]. The radiological diagnosis is based on the modified orientation of the band on plain abdominal X-ray, associated with an enlarged gastric pouch at the upper GI series [14]. Band position, connection tube location, and continuity with the access port should be always checked both on plain and contrast X-ray. Emergency treatment consists in complete band deflation through the subcutaneous port system, nasogastric tube positioning in the pouch (possibly under radiographic control), and intravenous administration of fluids, antiemetics, and proton pump inhibitors [5, 8, 9]. This should determine a significant improvement of the condition and allow time to refer the patient to the bariatric center. Complete deflation should be done under strict aseptic conditions. After having determined the port location either by palpation or fluoroscopy, a non-coring Huber needle is smoothly introduced percutaneously through the dome of the port until the metallic bottom is reached, and then the saline solution can be aspirated. Good results can be achieved with conservative treatment, especially in the symmetrical dilation, but if symptoms persist for more than 3–5 days, surgical treatment is needed to prevent gastric pouch ischemia. Laparoscopic approach in case of acute slippage is effective in over 95% of the cases and is the standard choice, provided that no gastric necrosis is found [5, 8, 9, 13].
2.2 Gastric Obstruction
Food bolus obstruction can be the cause of an acute, persistent dysphagia. It should be conservatively managed in the same manner as acute slippage. If band slippage is not confirmed by gastrografin swallow, band deflation, intravenous fluids, and even endoscopic removal of the bolus should be done. If the treatment is successful, the patient should be encouraged to obtain nutritional counseling and bariatric surgeon’s reevaluation [9].
2.3 Complicated Intragastric Band Migration
Intragastric band migration (incidence 0.8–4%) is usually diagnosed at the radiological or endoscopic follow-up and usually is not a surgical emergency [8, 10, 12, 15, 16]. Intraoperative gastric wall trauma and tight band placement may account for early erosion; high band pressure, band overinflation, and dietary noncompliance can cause late band erosion. Band removal is mandatory because of the risk of complications as hemorrhage or perforation but is part of a standard approach in a bariatric center, which includes serial upper GI endoscopies. Most cases do not require emergency surgery. Chronic melena, with chronic anemia, in the absence of abdominal symptoms and with a stop of weight loss or even weight regain, is a sign of latent band erosion and possible intragastric migration [6–10, 12]. Hemorrhage from an eroded band has been reported [17]. Port site infection might be a sign of band erosion into the gastric wall [8, 9]. Acute port infection, with evident local signs, like port site inflammation, abscess, or cutaneous fistulas, requires urgent surgical drainage and referral to the bariatric center for further investigations.
2.3.1 Roux-en-Y Gastric Bypass
Laparoscopic Roux-en-Y gastric bypass with isolated gastric pouch was described in1993 by Wittgrove et al. For a long time, the RYGBP has been the most largely performed bariatric/metabolic procedure in the USA (Fig. 2.2). The standard gastric bypass procedure consists in (I) creation of a small, (15–30 mL) isolated gastric pouch using an endoscopic surgical stapler, accompanied by a bypass of the remaining stomach, duodenum, and first tract of jejunum, and (II) reconstruction of the GI tract with the Roux limb with a biliary loop length of 30–75 cm and alimentary limb length of 100–150 cm. In the variant “long limb,” the length of the alimentary limb is 150–250 cm; in the “distal” RYGB, the common channel length is 150 cm, measured from ileocecal valve. The latter variant is more similar to the BPD inducing more intestinal malabsorption than standard LRYGB , which produces a limited malabsorption of around 30% of lipid. In a high-quality study, excess body weight loss at 1 year was 76% after standard RYGB. Blood pressure decreases significantly after this procedure, and it has been shown that at 1 year of follow-up, 46% of patients achieved complete resolution of hypertension, while 19% showed an improvement. The RYGB prevents diabetes in 99–100% of patients with impaired glucose tolerance and leads to clinical resolution of 80–90% of newly diagnosed cases of T2DM . Moreover, after RYGBP, a rapid improvement in insulin resistance with in few days has been described. After 8 years, RYGBP was associated with an EWL of 69%, hypertension, diabetes, dyslipidemia resolution rate of 66%, 82%, and 40%, respectively [18].


Fig. 2.2 Gastric bypass
Laparoscopic mini- (or single-anastomosis) gastric bypass is a new alternative to standard RYGB. Developed by Dr. Robert Rutledge in 1997, this procedure is becoming popular because of its simple surgical technique (single, gastrojejunal anastomosis) and preliminary results that reported a lower complication rate, a similar efficacy, including weight reduction and control of DM, compared to standard RYGBP.
2.4 Anastomotic Leak
Anastomotic leak after GBP is a life-threatening complication (incidence 0–6.1%) [9, 19]. It presents some problems: timing (early or late), clinical presentation (from subclinical to sepsis), diagnosis (gastrografin swallows, CT scan, and blood counts), and treatment (conservative, including fluid resuscitation, antibiotics, analgesia, endoscopic stent, and transfer to the bariatric unit when possible). Surgical emergency treatment should be considered in a hemodynamically unstable patient with severe, persistent symptoms: intense washout of the abdominal cavity and multiple drain placement should be considered. Laparoscopic approach is the best option if experience is present [9]. Final surgical treatment should be referred to the bariatric center.
2.5 Complicated Marginal Ulcer
Marginal ulcer is a peptic ulcer on the mucosa near the site of the gastrojejunal anastomosis. It can occur early (1–3 months) or late after a GBP. It is located either on the anastomosis (50%) or the jejunum (40%); its reported incidence ranges between 0.3 and 16%, and several risk factors are involved (operative technique, type of absorbable/nonabsorbable sutures used, patient age, history of previous gastric surgery, preoperative diabetes, coronary artery disease or peptic ulcer disease, and the use of nonsteroidal anti-inflammatory medications or tobacco) [20–22]. In a large cohort study, prior or current tobacco use remained the only independent risk factor for ulcer persistence after treatment [22]. The most common presenting symptom is pain (63%) followed by bleeding (24%), but perforation can occur. Perforated marginal ulcer incidence after GBP is ≤1%. The clinical picture is similar to any other visceral perforation: severe epigastric pain, tachycardia, fever, and leukocytosis, with free air on plain radiographs or CT scan. Surgical management is required; it can be performed by laparoscopy or laparotomy. It may require surgical revision of the anastomosis, with significant morbidity, but successful management with omental patch has been also reported [23, 24]. A gastrostomy tube in the excluded stomach should be considered for enteral nutrition, and high doses of PPI are always associated [9, 20, 25].
Stricture of the gastrojejunostomy after a Roux-en-Y gastric bypass is reported in 3 to 27% of cases. Quite often, however, the presence of a marginal ulcer can narrow the anastomosis or the efferent limb, causing symptoms of obstruction that can be mistaken for the result of a stricture of the anastomosis due to scarring [20, 24, 25].
2.5.1 Biliopancreatic Diversion
Scopinaro first performed the biliopancreatic diversion (BPD ) in 1976 in Genova (Italy). This operation induces controlled malabsorption without many of the potential side effects caused by bacterial overgrowth and indiscriminate malabsorption associated with the jejunoileal bypass, which is now completely abandoned. This operation combines removal of two thirds of the stomach (distal gastrectomy) with a long intestinal bypass (common channel 50 cm, alimentary limb 250 cm). The operation includes cholecystectomy and liver biopsy.
The procedure was later modified by Hess with a variant that he called “duodenal switch” in 1986 that was first performed laparoscopically by Gagner in 1999. Instead of performing a distal gastrectomy, a “sleeve gastrectomy ” along the vertical axis of the stomach (volume of remnant 70–150 mL) was proposed, preserving the pylorus and initial segment of the duodenum, which is then anastomosed to a segment of the ileum, similarly to the BPD , to create the alimentary segment (common channel 100 cm). Preservation of the pyloric sphincter is designed to be more physiological. The sleeve gastrectomy decreases the volume of the stomach and also decreases the parietal cell mass, with the intent of decreasing the incidence of ulcers at the duodeno-ileal anastomosis. These procedures produce selective malabsorption by limiting food digestion and absorption to a short, common ileal segment. The potential for nutritional complications exists. Patients undergoing the biliopancreatic diversion or duodenal switch procedure require close long-term medical follow-up and regular monitoring of fat-soluble vitamins, vitamin B12, iron, and calcium. Scopinaro et al. report the long-term outcome of BPD in a series of 312 obese patients with T2DM. Fasting serum glucose concentration fell to within normal values in all but two of the patients and remained in the physiological range in all but six, for a mean follow-up of 10 years [26]. Inabnet reported recently a hypertension and dyslipidemia resolution rate of 52.9 and 64%, respectively, after BPD-DS . In order to reduce operative morbidity and mortality in high-risk superobese patients, BPD-DS was divided in two stages: laparoscopic sleeve gastrectomy (LSG ) as first stage followed after 6–12 months and by second stage consisting in duodeno-ileostomy and ileo-ileostomy.
Biliopancreatic diversion along with its variations is the bariatric/metabolic procedure with the higher reported estimated weight loss. Patients require particular attention, especially in the emergency room setting, for the changes in their gastrointestinal (GI) anatomy and physiology following surgery [27, 28]. Early or late complications of BPD or DS are rare and often require the experience of a bariatric surgery team for their prompt resolution. Many complications that might necessitate a general surgeon’s emergency attention are complicated marginal ulcer (hemorrhage or perforation), bleeding, small bowel obstruction (SBO) due to internal hernia (biliopancreatic limb, alimentary limb, or the common channel) or incisional hernia, small bowel or gastric perforation, leak from staple line or anastomoses, intra-abdominal abscess, and anastomotic stenosis [28–30]. Specific late complications, even if not surgical, might be observed in an emergency setting: protein malnutrition (often not properly treated in a nonspecialized center), severe anemia, and Wernicke’s encephalopathy [27–29]. Stabilization of the patient is usually possible in order to transfer the patient to a specialized bariatric center. Particular attention should be addressed to appendicitis or cholelithiasis after BPD or DS. Initial BPD included cholecystectomy, appendectomy, and hepatic biopsy [31], but after the introduction of laparoscopy and technical evolution to DS, these procedures are no longer routinely performed.
2.6 Sleeve Gastrectomy
Results obtained in terms of weight loss and resolution of comorbidities after LSG encouraged and stimulated the diffusion of this operation inducing several authors to propose this procedure as a primary bariatric procedure. In fact, LSG is a technically simple surgical procedure with a low complication rate and negligible long-term nutritional deficiencies. The effect on weight loss and resolution of comorbidities have been attributed to the reduction of the gastric capacity.
(restrictive effect) and/or to the orexigenic and anorexigenic intestinal hormone modification (hormonal effect).
Laparoscopic sleeve gastrectomy (LSG) (Fig. 2.3) is today recognized as a stand-alone procedure that originates from the two-stage approach of the biliopancreatic diversion with duodenal switch (BPD-DS) . Early staple line complications are rare but most feared; bleeding and/or leaks are usually managed by the bariatric center in the immediate postoperative days. Depending on the local regional circumstances, more and more bariatric procedures, including sleeve gastrectomy, are performed nowadays on a very short hospitalization, with early discharge as standard of care. Therefore, the general surgeon can be confronted even with early complications like bleeding or acute leaks.


Fig. 2.3 Sleeve gastrectomy
2.7 Suture Line Leakage
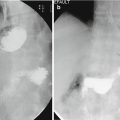
Suture line leakage rate after LSG ranges between 0.7 and 7%, depending on the series and the patient characteristics [32], with a risk ranging between 1.5 and 2.4% in recent systematic reviews and meta-analysis [33, 34]. Revisional surgery after initial bariatric procedure (conversion of gastric banding or vertical gastroplasty to LSG or gastric bypass) can increase the fistula rate up to 20% [32]. The critical areas for leakage are the top of the suture line, near the gastroesophageal junction (89%), and the transition point between sequential cartridges. Postoperative leaks may be classified into acute, late, very late, and chronic [32, 35, 36]. In a large retrospective study including 2834 patients, the leaks were diagnosed at a median of 7 days postoperatively, with 73% of the cases at 3–14 days after discharge [37]. Symptoms and signs suggestive of a localized or generalized peritonitis (pain, fever, tachycardia, tachypnea, often left pleural effusion, and pain in the left shoulder) in a patient who recently had bariatric surgery are likely due to a late fistula. Abdominal plain X-rays and contrast X-ray studies may assist in the diagnosis, but in all suspected cases, a CT scan with oral gastrografin is essential. Misdiagnosis will worsen the patient’s future evolution.
The CT scan usually shows three possible pictures:
- 1.
High staple line fistula (esophagogastric junction) along with a left subdiaphragmatic collection.
- 2.
“Bubbles” in the peri-gastric fat near the staple line and a peri-gastric fluid collection without evidence of contrast medium leak.
- 3.
Multiple leaks and diffuse fluid collection in the latter case; an emergency laparoscopy/laparotomy (according to the local skill) may be indicated to carry out a lavage of the upper abdominal cavity and drainage as a first emergency surgical step. Conservative treatment including bowel rest, fluid resuscitation, antibiotics, aspiration of esophageal and gastric secretions, nutritional support, analgesia, endoscopic stent, and transfer to the bariatric unit is appropriate, but experienced intensive care, endoscopy, and radiology units may be required. Surgical emergency treatment should be considered in a hemodynamically unstable patient with severe, persistent symptoms and an acute fistula or a late fistula with diffuse fluid collection. Laparoscopic approach is the best option if experience is available [9] and can accomplish extensive peritoneal washout, identification of the fistula site (check first the esophagogastric junction), and multiple drainage.
No attempt of correction of the staple line defect is usually indicated. Three main objectives are pursued: sepsis control, prevention of abdominal recontamination, and nutritional (parenteral and enteral) support [35, 38–40]. All other cases of late staple line fistula, if stable, should be referred to the bariatric center where the best management strategy can be adopted. Their treatment is based on percutaneous drainage plus parenteral/enteral nutrition and antibiotics. An endoscopic prosthesis can be positioned in selected cases and/or endoscopic fibrin glue applied [32, 41–44].
2.8 Mid-Gastric Stenosis
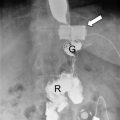
Persistent vomiting and food intolerance can be caused by a mid-gastric stenosis (incidence 0.7–4%, usually less than 1%) as a result of a sleeve gastrectomy calibrated on a too narrow tube or due to the oversewing of the staple line [14, 41]. After conservative treatment of dehydration, the patient should be referred to the bariatric center for endoscopic or radiological dilation (usually the patient requires three to four endoscopic/radiological outpatient sessions) [45]. Unsuccessful treatment might rarely determine an elective conversion to gastric bypass or seromyotomy for a long stenosis [14].
2.9 Gastric Plication
Gastric plication is an emergent bariatric operation that was re-proposed a few years ago, after its initial description three to four decades ago [46], and improved by the laparoscopic approach and recent experimental studies [47, 48]. Laparoscopic greater curvature gastric plication (LGP) is still an investigational procedure, popular in the Middle East and Central America [49].
In the LGP the dissection of the greater gastric curvature started 5 cm from the pylorus up to the angle of His, left undisturbed. A bougie was inserted into the stomach. Gastric plication was performed starting at the His angle. A running, extra-mucosal, nonabsorbable suture was performed as a first row. A second row of extra-mucosal, nonabsorbable, interrupted sutures was then added in order to tighten the plication.
Experience with management of complications is limited: the two systematic reviews, available to date, include only 307 and 521 patients [49, 50]; their data have to be interpreted with caution; the low follow-up rate of several published series may imply a selection bias, and complications may be underreported [49]. Average complication rate is 8 and 15.1%; reoperation rate is 4.6 and 3%. Prolonged nausea, vomiting, and sialorrhea are common and may require readmission for intravenous administration of antiemetics, prokinetics, and hydration [50, 51]. Reported major complications are gastric obstruction, bleeding (intraluminal upper GI bleeding or intraperitoneal), leaks, and perforations.
Gastric obstruction is the most common reason for reoperation. It is often due to adhesions and fold prolapse or edema, followed by serous fluid collection within the virtual cavity between the folds. It can occur either in the early postoperative period or several weeks after the operation [51], and it may require treatment by a non-bariatric team. Initial conservative treatment (anti-inflammatory and PPIs) can be attempted. If vomiting does not improve, gastroscopy could resolve the obstruction by fold manipulation, but laparoscopic partial or complete reversion of the plication will become necessary in case of refractory obstructive symptoms [52], and a referral to the bariatric center is recommended whenever possible. Acute gastric herniation through the sutures has also been described [49, 51]. Leaks after LGP are documented in 1.6% of patients [50]; they range from minor leaks to gastric perforation, determined either by the manipulation of the electrosurgery devices or inadequate technique, causing ischemia or stenosis of the gastric tube [48, 49]. Prolonged postoperative nausea and forceful vomiting may also be involved in a leak development. Treatment of peritonitis is indicated, with copious lavage of the abdominal cavity; suture invagination of a perforation within the stomach wall or partial or total reduction of the plication may be indicated.
2.10 Non-procedure-Specific Acute Complications
2.10.1 Bleeding
Bleeding can be a consequence of the staple line or other sources [10, 12, 17, 41]. Trocar site bleeding, splenic injury, or liver lacerations from retractor injury are rare but possible hemorrhage sources. Usually these complications appear in the first 48 h after surgery, when the patient is still under bariatric specialist surveillance, but routine early discharge policies can bring an early postoperative bleeding to the attention of a general surgeon or an emergency physician. Although the clinical picture of bleeding often leaves no room for doubts (anemia, hypotension, tachycardia, hematemesis, and melena), the site of bleeding and the corresponding control can be a challenge. Early bleeding from a staple or suture line can be extra- or intraluminal. Most early upper gastrointestinal, intraluminal hemorrhage will manifest with hematemesis and melena, and their treatment does not differ from any other upper GI bleeding in a non-bariatric patient. In all cases, management includes serial blood counts, good intravenous access, fluids administration, stop of anticoagulants, monitoring of vital signs, and upper GI endoscopy. If the endoscopist is familiar with the anatomic changes related to the bariatric procedure, endoscopy may reveal the bleeding point from the inner side of the staple line and control it by adrenaline injection, electrocoagulation, or endoclips if good visualization is obtained. The endoscopic examination for perforation at the bleeding site should be not omitted. Late bleeding in a gastric bypass can present relevant peculiarities. Heneghan et al. reported an incidence of 0.94% of 4466 patients who underwent GBP during a 10-year period [53]. Bleeding occurred within 30 days in 71% of the patients, and the etiology included staple lines, iatrogenic visceral injury, or mesenteric vessel bleeding. The authors report that 43% of the cases required operative intervention to achieve hemostasis. A significant amount of later hemorrhage in a gastric bypass is related to a marginal ulcer. Severe hemorrhage or perforation can be faced by a general surgeon as reported [9, 20]. Endoscopic management is essential, and only its failure can indicate an angiography (selected cases) or surgical exploration. The jejuno-jejunal anastomosis of a gastric bypass or the ileoileal anastomosis in a biliopancreatic diversion can also be responsible for bleeding. Spiral angio-CT scan [28] or selective angiography can assess bleeding at these sites. Bleeding in a GBP can originate also from erosion or ulcerations of the gastric remnant [54] or even from duodenal or jejunal ulceration [55]. Refractory bleeding from the gastric remnant or other sites with no access for endoscopy can entail surgical revision [9, 20, 25]. Upper gastrointestinal bleeding can occur anytime after LAGB positioning due to erosions or ulcers. Peptic ulcer, Mallory-Weiss tear, erosive gastritis, and esophagitis can also be sources of bleeding in patients with LAGB. Acute upper GI bleeding, occurring in late follow-up, could be the result of an active ulcer, and careful endoscopy should recognize and even treat it [8–10, 12]. An extremely rare cause is bleeding from a peptic ulcer during pregnancy [8, 9] due to severe eclampsia or preeclampsia and stress that may lead to acute gastric or duodenal ulceration, even complicated with perforation. Severe vomiting in pregnant patients with a gastric band could induce peptic ulcer; band deflation at the beginning of pregnancy in anticipation of pregnancy-induced vomiting seems advisable, even if there is a risk of excess weight regain; however, most series report a selective deflation policy [56]. The initial treatment of upper GI bleeding after LAGB, as of any other gastrointestinal bleeding, is conservative (adequate resuscitation, close monitoring, assessment of the severity of bleeding, blood transfusions, and emergency endoscopy when necessary). When surgery becomes necessary, the patient should be referred to the bariatric center, when the clinical situation permits it. Extraluminal bleeding could be shown by the drain when present and still functional; otherwise, an acute drop of hematocrit, with hypotension and tachycardia, would indicate unstable hemodynamic condition that may require reoperation for lavage, identification of the source, and hemostasis. At surgery, the bleeding source (staple line, retrogastric vessels, short gastric vessels, omentum dissection line, splenic or liver injury, trocar site, etc.) will be often no longer active; intense abdominal washout, multiple drainage, and supportive intensive care will suffice. Laparoscopic approach is recommended, but only where experience is available.
2.10.2 SBO After Bariatric Procedures
Evaluation and treatment of SBO is one of the most common tasks that a general surgeon or an emergency physician has to face. About 16% of surgical admissions and more than 300,000 operations annually in the USA are related to SBO [57].
The standard management algorithm, commonly practiced for SBO, includes an initial trial of non-operative treatment using nasogastric decompression, bowel rest, fluid resuscitation, and close monitoring. A substantial number of cases are treated only with such conservative measures in the absence of signs suggesting impending or ongoing bowel ischemia [57, 58]. In patients with history of bariatric surgery, the outcome of commonly adopted protocols could be affected by several factors related to the new anatomy and physiology of the gastrointestinal tract. Physicians that are not adequately familiar with these alterations may be misled in their evaluation. The nasogastric decompression may be ineffective on a substantial portion of the gastrointestinal tract (gastric remnant, biliopancreatic limb), and prolonged non-operative management may be futile and dangerous. If a Roux reconstruction is present, a portion of the bowel is excluded from the alimentary flow; the evaluating physician must consider that obstipation may then be absent even in a complete obstruction and that the risk of a closed-loop bowel obstruction is higher than in non-bariatric patients. Finally, it may be difficult to identify small incisional hernias (trocar site hernias) in an obese patient, and the incidence of internal hernia is higher. The worldwide increasing diffusion of bariatric surgery makes it crucial that any physician involved in emergency care becomes familiar with the peculiarity of SBO in the bariatric patient and achieves a complete understanding of the bariatric procedures. The incidence of SBO after open bariatric surgery has been reported to be in the range of 1–5% [59]. Smith et al. [60] reported an incidence of 2.7% after laparoscopic gastric bypass. A recent review of nearly 10,000 laparoscopic gastric bypass reported an overall incidence of 3.6%. Martin et al., analyzing the Nationwide Inpatient Samples (2006–2007), reported 9505 admissions for SBO in bariatric patients vs. 54,342 in the non-bariatric population. Surgery was performed in 62% of the patients versus only 28% of the non-bariatric group. Bariatric patients were also taken to the operating room earlier (1 vs. 3.3 days). These data emphasize the necessity of earlier emergency surgery to avoid severe intestinal complication and related mortality in this cohort of patients. It appeared also that bariatric patients are approached more often by laparoscopy with good outcomes and significantly less complication and mortality [61]. Small bowel obstruction has been anecdotically reported after LAGB due to adhesions in patients with history of multiple surgical interventions and also due to the connection tube or to the abnormal migration of an eroded band [8, 15, 62, 63]. Diagnosis is not always easy because patients with LAGB may be unable to vomit; liquid accumulation within the closed loop determines severe gastric dilation that can cause gastric wall necrosis [15]. Early diagnosis of small bowel obstruction and early intervention, which can be as straightforward as fluid removal from the LAGB and nasogastric tube insertion, are of utmost importance. Failure of accomplishing these basic steps can determine an unfavorable prognosis, with evolution toward stomach or bowel necrosis. The most common cause of SBO in the bariatric population is an abdominal wall or internal hernia [61]. Port site hernia could be determined by the ≥10 mm trocar abdominal fascial defects left unclosed at the end of the laparoscopic bariatric procedures [9, 10, 12]. A trocar site hernia is an uncommon complication of laparoscopic surgery; however, it is necessary to take into consideration this possibility in the bariatric patient: a recent review showed that higher BMI is a significant risk factor for its development [64] even if its incidence after bariatric surgery does not seem to be higher [65]. The identification of small incisional hernia can be exceedingly difficult in obese patients. Emergency treatment for partial or complete bowel obstruction allows rapid reduction of the herniated content. A laparoscopic approach is recommended if adequate experience is available; bowel resection might be necessary in case of perforation or bowel ischemia. The closure of the abdominal wall defect completes the operation. Internal hernia is widely recognized as the most frequent cause of SBO (>50%) in bariatric patients [66]. SBO after GBP or BPD is determined mainly by internal hernia [27, 28, 67, 68]. There are three classic locations where SBO can occur after GBP : Petersen space (between Roux limb’s mesentery and transverse mesocolon), at the transverse mesocolon defect (for retrocolic bypasses), and at the jejuno-jejunostomy [67]. Obstruction can involve the alimentary limb, the biliopancreatic limb, or the common channel, with incidence between 0.4 and 7.5% [68, 69]. Symptoms can suggest the site of obstruction: heartburn and vomiting are associated with the common channel or alimentary limb’s obstruction; bilious vomiting originates from the common channel obstruction; distension of the gastric remnant or biliopancreatic limb suggests common channel and biliopancreatic limb obstruction. Diagnosis is based on clinical presentation, plain abdominal X-ray, and upper gastrointestinal studies. CT scan is a standard diagnostic tool and can demonstrate the dilatation of the Roux limb, of the gastric remnant, or of the biliopancreatic limb, depending on localization [70–73] . Even sophisticated imaging (multislice CT spiral scan), however, will fail to disclose internal hernia in up to two of three cases [74, 75]. This has led to an increasing acceptance for immediate laparoscopic/laparotomic exploration in bariatric patients with subtle symptoms of SBO [67, 76, 77]. Symptom persistence, acidosis, lactate rise, or signs of an acute abdomen should prompt exploration. Laparoscopy is the best choice (if previous bariatric surgery was also laparoscopic) where expertise is available. Small bowel assessment and handling are not easy, regardless the access. As in any laparoscopic exploration for SBO, a retrograde examination of the bowel starting from the ileocecal valve is easier and less risky. In case of positive identification of an internal hernia, a gentle reduction should be done, followed by the closure of the mesenteric defect. Patients with history of bariatric procedures, who also had other abdominal surgery (cholecystectomy, incisional hernia repair, gynecological operation, etc.), should always be checked for potential mesenteric defects in other areas. Symptoms can also evolve chronically, with intermittent and recurrent abdominal pain associated with nausea and vomiting but without a clear obstructive picture. This can be misinterpreted as food intolerance, marginal ulcer, or gastroesophageal reflux disease (GERD). Quite often, the intermittent pinching of a loop of bowel in an internal hernia defect can induce chronic, intermittent abdominal pain; the mechanism underlying the symptom may remain unknown, not discovered even by the most sophisticated imaging techniques, unless a very high degree of suspicion is maintained. Any patient with previous GBP or BPD who presents with chronic, intermittent abdominal pain or recurrent signs of a SBO should be suspected of having an internal hernia, and a referral to a bariatric center for a laparoscopic exploration may be warranted. Early diagnosis and intervention are imperative in order to reduce morbidity and mortality associated with intestinal necrosis [31, 67, 73].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree