While well-differentiated thyroid cancer is generally thought to be a treatable cancer with excellent outcomes, some patients suffer from recurrent disease. Risk factors for recurrent disease include primary disease greater than 4 cm, incomplete resection, multiple positive lymph nodes in the central compartment, and lateral neck disease with multiple positive lymph nodes in multiple levels or pathologic extracapsular extension. These factors can help stratify the thyroid cancer population in to low-, medium-, and high-risk patients. Low-risk patients can generally be followed with thyroglogulin levels and routine ultrasounds to the head and neck. High-risk patients are best monitored with stimulated thyroglobulin, ultrasound of the head and neck, and low-dose iodine 131 uptake scans at the 6- to 12-month mark. The treatment of locoregional recurrent thyroid cancer is surgical resection with the overall goal of complete tumor removal while maintaining function and decreasing risks. The use of adjuvant therapy is dependent on the presence / absence of high risk pathologic features.
The prognosis for differentiated thyroid cancer is generally considered excellent with 10-year survival rates greater than 90%. There is widespread agreement that treatment should include surgical thyroidectomy. However, debate continues about the extent of the primary surgery. It is generally accepted for thyroid cancer that clinicians need to assess not only the thyroid gland, but also the regional lymphatics either clinically, radiographically, or intraoperatively. With lymph nodes being palpably present in papillary thyroid carcinoma in up to 15% to 40% of cases and occult in up to 80% of cases, it is important to diagnose those patients who are at risk for regional metastasis. The treatment of the neck in clinically node-positive patients and in high-risk patients is surgery, including the appropriate neck dissection, either central compartment or lateral, depending on the location of the at-risk lymph nodes. If the lateral compartment is involved, ipsilateral central compartment surgery should be considered. Adjuvant therapy with radioactive iodine ablation is considered for high-risk patients, including known residual disease in the thyroid bed, younger patients with distant metastasis (stage II), older patients with large tumors (larger than 1 cm), and is considered for most patients with multifocal disease, nodal metastasis, extrathyroidal or vascular invasion, and aggressive histology.
Despite an aggressive approach to primary differentiated thyroid cancer, a certain percentage of patients develop recurrent disease (5%–20% in papillary and 25%–73% in follicular and Hürthle cell). This can occur either in the thyroid bed or in the lymph nodes, centrally or laterally. Because of this propensity for recurrence, it is important to identify which risk factors are involved in recurrent disease, determine how to identify and diagnose recurrent disease, and discuss the treatment options.
Risk factors
Recurrent locoregional disease for differentiated thyroid cancer is relatively common with rates ranging from 5% to 70%. However, despite this, the overall disease 10-year survival is above 90%. This creates a diagnostic and treatment dilemma: How important is it to pursue this disease aggressively when there is already such an excellent long-term outcome? To answer this question, it is necessary to better understand the effect of recurrences on survival and risk of death.
The impact of the development of recurrent disease on long-term survival has been controversial. It is felt that the development of locoregionally recurrent disease increases the risk of death from differentiated thyroid cancer fivefold. Other reports say that while regional recurrence does not adversely affect survival, the development of locorecurrence (primarily thyroid bed) does increase the possibility of tumor-related death in up to 50% of cases. Because of this effect on overall survival, it becomes important to identify the risk factors that may influence the development of recurrent disease.
Risk Factors for Local Persistent or Recurrent Disease
In patients who develop local disease recurrence, the majority (>50%) of them have stage III (tumors >4 cm) on presentation and are over the age of 45. The type of surgery for the primary disease (hemi-, subtotal, or total thyroidectomy) does not have an impact on the development of thyroid bed recurrences. The development of local recurrence is primarily related to the ability to achieve complete surgical removal of the tumor, regardless of which surgery is used. The presence of central compartment lymph nodes also increases the potential for persistent disease up to 27%.
Risk Factors for Regional Persistent or Recurrent Disease
The development of regional disease after treatment of the primary thyroid cancer can occur in up to 15% of patients. Lymph node metastasis has traditionally not been associated with death from thyroid cancer. However, it is important that the lymph node metastasis be identified during the initial evaluation, and surgical removal at the initial procedure may improve survival. The risk factors for the development of persistent or recurrent disease in the neck include multiple metastatic lymph nodes and extracapsular extension. As reported by Leboulleux and colleagues, when a tumor is less than 4 cm but there are more than 10 metastatic lymph nodes or more than 3 lymph nodes with extracapsular extension, the risk of persistent disease is 20% to 45%. When the metastatic tumor extends above 4 cm, the risk increases to 75%. Recurrent disease follows the same trend, with increased incidence of recurrence when the thyroglobulin is increased and there are multiple lymph nodes and extracapsular extension.
Risk Factors for Decreased Survival
While deaths from differentiated thyroid cancer are rare, some risk factors associated with such deaths can be identified. As discussed in a large matched-control study, the most important risk factor is the completeness of surgical excision of the primary. Incomplete resection of the primary tumor increases both the risk of locoregional recurrence and ultimately the risk of death. In stage III disease, a subtotal resection has a 50% increased risk of death compared with those receiving a total thyroidectomy. However, the extent of lymph node surgery has no impact on overall survival: Those patients receiving extensive modified radical neck dissections tend to have a worse prognosis than those undergoing a lymph node “plucking” procedure, but that prognosis is directly related to increased stage of the disease. The type of adjuvant therapy (radioactive iodine or external beam radiation) does not have any impact on the overall survival of the patients.
As discusses in a large match-control study from Denmark, the overall tumor stage (T4) as well as the diagnosis of distant disease and advanced age are associated with increased risk of death from differentiated thyroid cancer. The overall 5-, 10-, and 15-year survival rates for the patients with T1 through T3 disease is 96%, 93%, and 87%, respectively, while those with T4 disease is 70%, 54%, and 45% respectively. Even more dramatic is the overall survival for the patients with distant disease: 56%, 32%, and 32%.
Diagnosis of recurrence
After the initial treatment of differentiated thyroid cancer, the patients need long-term follow up and management. Because recurrence of disease is associated with a less favorable prognosis, it becomes important that there be reliable methods for accurate surveillance directed at identifying recurrence in both the primary thyroid bed and the regional nodal basins. The recommended method of follow-up depends on the level of risks for recurrence: low, intermediate, or high. Low-risk patients include those with complete tumor resection and with no local or distant metastasis, no tumor invasion locoregionally, no aggressive histology, no vascular invasion, and no iodine 131 ( 131 I) uptake outside the thyroid bed. The intermediate-risk patients have microscopic extrathyroid invasion, aggressive pathology, and vascular invasion. High-risk patients have macroscopic tumor invasion, incomplete tumor resection, distant metastasis, and 131 I uptake outside the thyroid bed ( Box 1 ).
Low risk
No tumor capsule invasion
Complete tumor resection
No vascular invasion
No aggressive histology
No 131 I uptake outside thyroid bed
No distant metastasis
Intermediate risk
Microscopic extra thyroid invasion
Vascular invasion
Aggressive pathology
High risk
Macroscopic tumor invasion
Incomplete tumor resection
131 I uptake outside the thyroid bed
Distant metastasis
Thyroglobulin
Measuring serum thyroglobulin levels is the primary modality of monitoring patients for recurrent or persistent thyroid cancer. Generally, thyroglobulin is a very sensitive and specific method for detecting recurrences. However, differentiated thyroid cancer patients are usually treated with thyroxine replacement, suppressing the thyrotropin below 0.1 mU/L. When thyrotropin is suppressed, the thyroglobulin level is less reliable as a method for detecting small thyroid recurrences. To identify a small recurrence, thyrotropin-stimulation serum thyroglobulin measurements may be required. In addition, if the patient has antithyroglobulin antibodies, the thyroglobulin becomes less sensitive to detect recurrent cancer. While the normal level of thyroglobulin differs for each patient, it is generally accepted that a level above 2 ng/mL is associated with recurrent disease.
Whole-Body Iodine 131 Uptake Scan
A whole-body 131 I uptake scan is typically used as a part of the radioactive iodine ablation treatments to determine the accuracy of the ablative procedure. After radioiodine, the whole-body scan is a low sensitivity test (20%) and it is not typically used in low-risk patients, especially if the thyroglobulin is undetectable and the ultrasound is negative. In high-risk patients, a low-dose 131 I uptake scan might be useful in evaluations for persistent disease at the 6- to 12-month mark. In the high-risk patient, the low-dose 131 I uptake scan, when combined with a stimulated thyroglobulin, offers a sensitivity for identifying recurrent disease approaching 100%.
Cervical Ultrasound
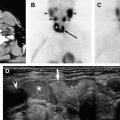
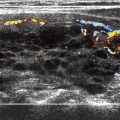
Routine use of high-resolution ultrasound is becoming the main method for postoperative thyroid bed and cervical lymphatic surveillance. As the expertise in this method has improved and as clinicians have become more familiar with the characteristic findings of the postsurgical bed, the sensitivity and specificity of this imaging modality has become very reliable. It is easy to perform and the cost is relatively low. In addition, the ability to perform immediate diagnostic fine needle aspiration on any suspect abnormality makes this a very favorable technique. It is possible to identify recurrences that are as small as 3 to 5 mm. Radiographically, the finding of hypoechoic or cystic mass within the thyroid bed is highly suggestive of recurrence. The identification of further details, such as marginal irregularity, microcalcifications, and taller dimensions, make it possible for ultrasound, in combination with the thyroglobulin test, to deliver a sensitivity of 96% for finding recurrent disease, as compared to 85% when the stimulated thyroglobulin test is used alone. The finding of hypervascular lymph nodes is also very sensitive.
CT
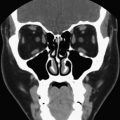
While ultrasound has been traditionally the imaging modality of choice for evaluating the neck for residual or recurrent thyroid cancer, the high-resolution, multislice CT scan can be an important imaging modality for this disease process. It can help identify the location of recurrences and clearly define the anatomic relationships of the paratracheal region, vasculature, and mediastinum. Even more detailed than the standard CT scan, the four-dimensional (4D) CT has been used to offer even more detail to location of the recurrent tumor. The 4D CT scan offers the added dimension of showing changes in perfusion of the contrast over time. The high-resolution images produce excellent documentation of the tumor location and are easily interpreted by surgeons used to reading cross-sectional imaging. The 4D CT scan has been used in parathyroid localization and has proven to be quite useful in delineating the various structures in the thyroid bed and paratracheal region.
The negative aspect of CT is the use of iodinated contrast dye. This iodine load can alter radioactive iodine uptake for 6 weeks. However, in the face of postsurgical thyroid bed recurrences, the benefit of the superior anatomic localization of the 4D CT scan may outweigh the delay in radioiodine administration in regards to successful identification on the tumor in high-risk recurrent thyroid cancer patients. The unenhanced CT scan of the chest can be very sensitive to identify parenchymal disease.
MR Imaging
MR imaging has been an alternative to the iodine-contrasted CT scan for imaging in thyroid cancer. As a cross-sectional imaging study, it has a high sensitivity and accuracy for diagnosing metastatic cervical lymph nodes (95% and 83%, respectively). However, the specificity is only 51% for differentiating between malignant and benign lymph nodes. The use of an MR image for recurrent thyroid cancer can offer excellent information regarding the tumor location and size. However the downsides of MR imaging include the motion artifact that may occur in the paratracheal region, due to respiration; the long length of the study; and the high cost. There is little evidence that imaging with CT or MR image scans in a low-risk primary thyroid cancer increases survival and improves prognosis. However, in the recurrent thyroid cancer patient, the benefit of better tumor localization probably allows for more directed and complete pretreatment planning and surgical procedures.
Fluorine 18 Fluorodeoxyglucose Positron Emission Tomography
In recurrent thyroid cancer patients, the radioactive whole-body scan can be negative in 10% to 15% of patients with detectable serum thyroglobulin levels. This is either because the tumor volume is too small to be seen on iodine scan, or because the thyroid tumor cells are no longer picking up iodine. Because malignancy usually demonstrates increased glucose metabolism, it is likely that the recurrent thyroid cancer lesions that are no longer iodine avid can be visualized on positron emission tomography (PET–CT) scan. Several studies have demonstrated sensitivities of PET–CT scanning for identifying recurrent or metastatic thyroid cancer in the range of 66% to 79%. The combined PET–CT scan is most useful in the situation of low thyroglobulin (<10 ng/mL) and poor or no iodine concentration on whole-body imaging. When the PET–CT scan is positive, it can be very helpful in localizing the area of recurrent tumor and assist in surgical planning.
However, PET–CT scan is not useful as a screening tool because the false-negative rates are relatively high (15%–30%). Instead, the PET–CT scan should be used as an adjunct to the conventional imaging (ultrasound and whole-body scan) and fine needle aspiration. In addition, the PET–CT scan has been accurate in diagnosing subtle lung metastasis (>6 mm) and should be considered when there is elevated thyroglobulin and no iodine uptake on whole body imaging. See Table 1 for summary.
Diagnosis of recurrence
After the initial treatment of differentiated thyroid cancer, the patients need long-term follow up and management. Because recurrence of disease is associated with a less favorable prognosis, it becomes important that there be reliable methods for accurate surveillance directed at identifying recurrence in both the primary thyroid bed and the regional nodal basins. The recommended method of follow-up depends on the level of risks for recurrence: low, intermediate, or high. Low-risk patients include those with complete tumor resection and with no local or distant metastasis, no tumor invasion locoregionally, no aggressive histology, no vascular invasion, and no iodine 131 ( 131 I) uptake outside the thyroid bed. The intermediate-risk patients have microscopic extrathyroid invasion, aggressive pathology, and vascular invasion. High-risk patients have macroscopic tumor invasion, incomplete tumor resection, distant metastasis, and 131 I uptake outside the thyroid bed ( Box 1 ).