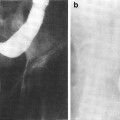
Fig. 1
Biocontinuum of adverse and late effects for the skeletal muscle (with permissions from Rubin and Casarett 1968)
2 Anatomy and Histology
2.1 Anatomy
2.1.1 Endochondral Bone
Evidence shows that skeletal development and volume depend on interacting genetic, racial, anthropometric, nutritional, and lifestyle factors that contribute together to the acquisition of peak bone mass, usually by the end of normal adolescence and puberty (Sheth et al. 1996). Hormones appear to play a leading role in developing and preserving skeletal health.
The human body consists of long, short, flat, and irregular types of bones. In the adult skeleton, which is divided into axial (skull, vertebrae, pelvis, sternum, and the ribs) and appendicular (limbs) parts, there are about 200 distinct bones. The long bones (e.g., femurs, tibias, and humeri) are found in the limbs and create a system of levers to allow locomotion (See Fig. 2 for anatomy of a long bone). The short bones compose the part of the skeleton mainly intended for strength and many limited motions. The flat bones (e.g., skull, scapula, sternum, and ribs) protect certain cavities, are composed of two thin layers of compact tissue, and enclose only a small amount of cancellous tissue. The irregular or mixed bones (e.g., vertebrae, sphenoid, and maxillary) are also composed of outer dense layers and filled with spongy cancellous tissue. Their diversity in shape and composition allows these bones to implement various essential locomotive and structural functions in the body.
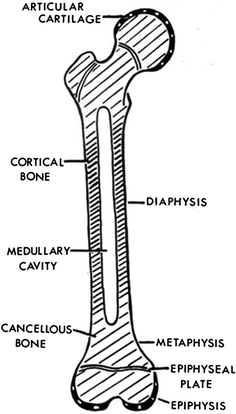

Fig. 2
Schematic of a growing long bone. The shaft of the bone is called the diaphysis, which contains the medullary cavity filled with bone marrow. The two expanded ends are the epiphyses. The epiphysis at each end extends from the articular cartilage to the epiphyseal growth plate. The metaphysis is the region between the epiphyseal plate and the diaphysis (with permission from Salter 1983)
2.1.2 Striated Muscle
Muscles are also widely distributed thorough the body and are connected with bones, cartilage, ligament, and skin either directly or through structures called tendons or aponeuroses. Muscles vary in form and size and can be elongated, broad, or flattened. They are classified as skeletal, cardiac, or smooth. Their function is to produce force and cause motion either for locomotion of the organism itself or movement of internal organs. Cardiac and smooth muscle contraction (such as peristalsis) occurs without conscious thought, but is essential for survival. Skeletal muscles or “voluntary muscles” respond to conscious control and are used to move the body and maintain posture. Skeletal muscle constitutes a large percentage of total body mass and varies by age and gender. Both cardiac and skeletal muscles are striated, but this chapter will confine itself to skeletal muscle.
2.1.3 Joints
The bones in the skeleton are connected by joints, some of which, like the facial bones, are immovable. These are joined with a thin layer of ligament or cartilage. In a joint where only slight motion is required, the bones are connected by tough and elastic fibro-cartilage. In a movable joint, the ends of the adjoining bones are usually covered with cartilage and held together by strong bands or capsules of fibrous tissue called ligaments. Movable joint surfaces are also at least partially covered with a fluid-secreting synovial membrane. Based on their function, joints are classified as synarthrotic (permitting little or no mobility), amphiarthrotic (permitting slight mobility), and diarthrotic (permitting a variety of easy movements). Joints can also be classified based on their anatomy or on their biomechanical properties. Joints allow movement and provide mechanical support.
2.2 Histology
2.2.1 Endochondral Bone
The long bones of the appendicular skeleton are composed of a middle portion of the shaft called the diaphysis and two enlarged rounded ends called epiphyses. The diaphysis is made up of cancellous bone and contains a medullary cavity filled with bone marrow and fat. The external layer of cancellous bone contains red bone marrow where the production of blood cellular components takes place. The cortical bone forms the outer layer of long bones and is the densest part of the bone, providing the main structural support of the human skeleton. The metaphysis, a small part of the bone between the epiphysis and the diaphysis where longitudinal growth of the bone occurs, contains the epiphysial growth plates. The outer layer of the bone, the periosteum, consists of dense connective tissue. Unlike osseous tissue, the periosteum has nociceptor nerve endings, which are nerve fibers that respond to noxious stimuli including pain.
The main histological functional subunit for both compact and cancellous bone tissue is a circularly appearing structure called the Haversian system or osteon. It contains a centrally located Haversian canal and concentrically arranged rings called lamellae. The osteocytes in their lacunae are also arranged concentrically and connected by canaliculi containing fibrils that allow the osteocytes to communicate with each other.
Long bones are formed by endochondral ossification. Ossification centers appear within a cartilaginous matrix in predictable order and timing during fetal and postnatal life. Some bones are ossified from a single center (e.g., the bones of the wrist and ankle), while others are ossified from several separate foci. At varying times after birth, secondary ossification centers develop in the cartilaginous end of the bone. Longitudinal growth of a long bone comes from the growth plate. The growth plate can be divided into five zones: (1) a zone of reserve containing resting chondrocytes; (2) a zone of proliferation containing chondrocytes undergoing mitosis; (3) a zone of hypertrophy containing hypertrophic chondrocytes secreting phosphatase; (4) a zone of calcification containing dead chondrocytes and calcified cartilage; and (5) a zone of ossification containing osteoprogenitor cells differentiating into osteoblasts (Fig. 3a, b). Although the reserve cells may serve a nutritional or storage function, their role is not well understood. It has been suggested that these cells may be recruited to repopulate the proliferative zone following damaging insults such as irradiation.
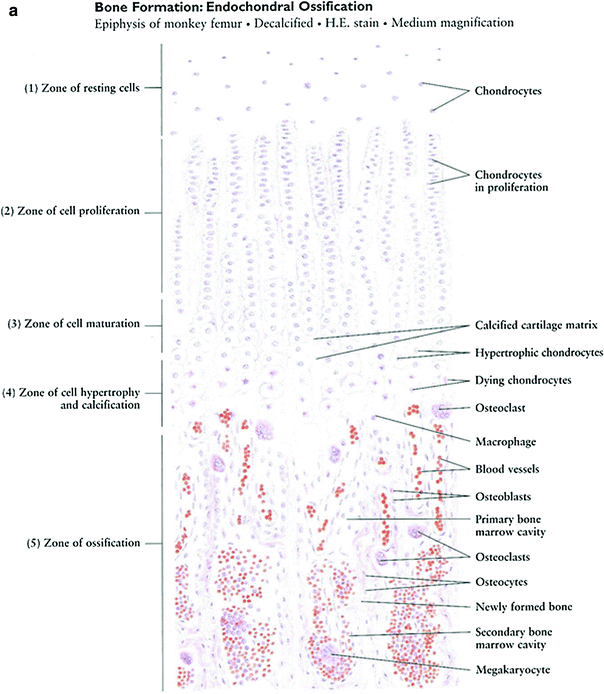
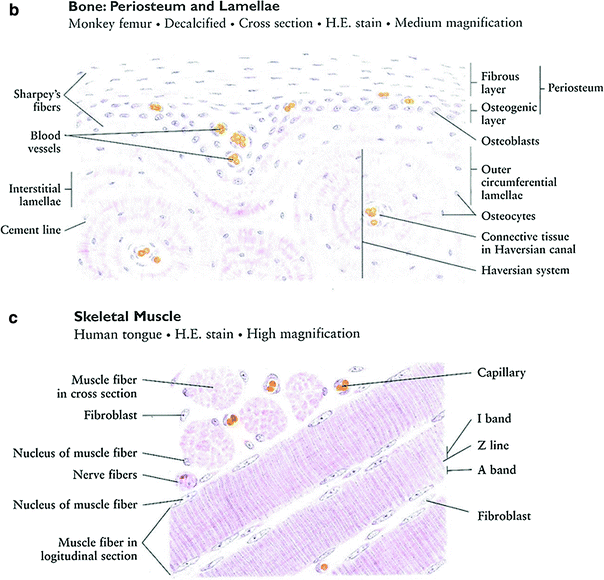


Fig. 3
a Bone formation: endochondral ossification. b Intramembranous bone formation. c Skeletal muscle (with permission from Zhang 1999)
2.2.2 Striated Muscle
Striated-muscle tissue is derived from the mesodermal layer of embryonic germ cells (McKinnel and Rudnicki 2005). Muscle cells contain contractile filaments called myofibrils, which are composed of sarcomeres, which move past each other and change the size of the cell. Actin (thin myofilament) and myosin (thick myofilament) are the main parts of sarcomeres and are responsible for forming cross-bridges for contracting and stretching (Fig. 3c). The myofibrils are contained with muscle cells (myoblasts and myotubes). Differentiated-muscle cells (myotubes) proliferate slowly, if at all. Skeletal-muscle cells are arranged in discrete muscles and individual muscles are connected by tendons to processes of the bony skeleton.
Skeletal muscle is divided into subtypes: Type I, slow oxidative or “red” muscle, is dense with capillaries and rich in mitochondria and myoglobin; Type II, “white fibers,” is less dense in mitochondria and myoglobin. Cardiac and skeletal muscles are striated, but anatomically different.
2.2.3 Joints
The layer of compact bone which forms the end of the long bone (the articular surface), and to which the cartilage is attached, is called the articular lamella. It is extremely dense and lacks Haversian canals. Cartilage is divided into hyaline cartilage, fibrocartilage, and yellow or elastic white fibrocartilage. Hyaline cartilage is covered with epichondrium, a dense connective tissue, except over the end of the articular lamella. White fibrocartilage consists of a mixture of white fibrous and cartilaginous tissue. It can form interarticular plates (menisci), connecting fibrocartilage (between vertebrae and other less mobile joints), circumferential fibrocartilage surrounding margins of certain joints (glenoid cavity of the shoulder), and stratiform fibrocartilage.
Ligaments consist of bands of connective tissue linking together articular bones and are mainly composed of white fibrous tissue. They are very flexible to allow freedom of movement, but simultaneously extremely strong. Another component of the joint structure is the synovial membrane, which is a thin, delicate layer of connective tissue. It secretes a thick, viscous liquid called synovial fluid that lubricates the joint, producing less friction with movement. Beneath the synovium is a layer of loose connective tissue with few cells called the subsynovium.
3 Physiology and Biology
3.1 Endochondral Bone Functional Subunits
Bone provides both a supportive and a protective function in the human body. Three primary types of cells produce and maintain bone tissue: osteoblasts, osteoclasts, and osteocytes. Osteoblasts are derived from osteoprogenitor cells, the progeny of mesenchymal stem cells (MSC). Osteoblasts secrete osteocalcin and alkaline phosphatase producing a mineral matrix of collagen. Clinical markers for osteogenesis and bone repair are serum alkaline phosphatase and serum osteocalcin. Once an osteoblast becomes completely encased within the matrix it becomes an osteocyte, though it still participates in minor bone remodeling. Osteocytes occupy a space called a lacuna and communicate with each other using long cytoplasmic filaments that traverse the bone in tiny canals called canaliculi. Neither osteoblasts nor osteocytes are mitotically active. Osteoclasts are derived from granulocyte-monocyte progenitor cells within the bone marrow. They are also nonmitotic and are responsible for bone resorption. Clinical markers of bone resorption are: urine hydroxyproline, urine pyridinoline cross-links, and serum N-telopeptides.
Several hormones have a clear influence on bone formation and remodeling, including growth hormone (GH), parathyroid hormone (PTH), calcitonin, thyroid hormones, androgens and estrogens, and cortisol. Vitamin D acts directly on osteoblasts to secrete IL-1, which stimulates osteoclasts to increase bone resorption.
3.2 Muscle Functional Subunits
Striated muscle contains bands composed of thick and/or thin myofilaments, which are the histologic functional subunit. Myofilaments are made from various forms of proteins (F-actin, myosin, tropomyosin, troponin, and titin), each responsible for the formation of cross-bridges, which produce the contracture and stretching functions of the striated muscles. This process is triggered from impulses coming from a neuromuscular junction transmitting signal via nicotine acetylcholine receptors (nAChR), ultimately causing interdigitation of the myofilaments. Several pharmacological agents (tubocurarine, vecuronium, etc.) as well as botulin toxin can disrupt the neuromuscular junction and paralyze muscles.
3.3 Bone Modeling
The concept of bone modeling is a complex dynamic process that needs to be defined logically to understand the radiation effects across the age spectrum from child to adolescence to mature adult. The dysplastic alterations are a function of the segment in a bone, the type of bone and the age at the time of irradiation. The anatomic-physiologic correlations are shown histologically with vectors of bone growth and modeling (see Fig. 4a, b for the anatomic-histologic correlation present in the ends of growing long bones).
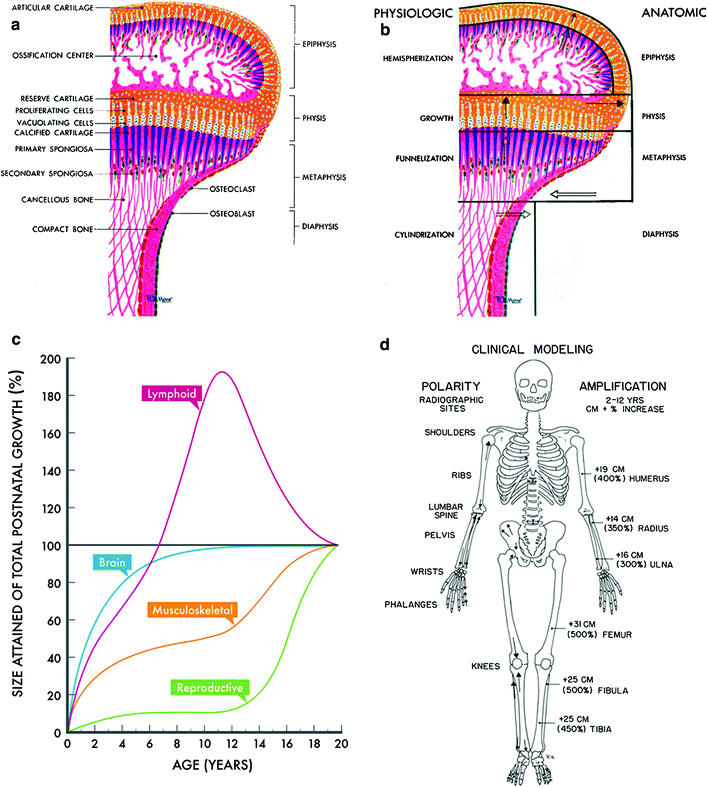

Fig. 4
Bone modeling. a Anatomic-histologic correlation of the end of a growing long bone: epiphysis, physis, metaphysis, and diaphysis. b Bone modeling at the same end of a growing long bone. (with permissions from Rubin 1964). c Post natal growth curves according to age and organ size. (Adapted and recreated from Tanner JM 1962) (Biopediatric chapter F2). d Polarity and amplification of skeletal growth and modeling. Arrows indicate polarity of growth, and percentages quantify the amplification from birth to adulthood (with permissions from Rubin 1964)
Epiphyseal segment or epiphysis. The epiphysis grows in the form of a hemisphere from the subarticular cartilage zone; thus the term hemispherization is used. It is recognized that the ultimate shape of any epiphysis is rarely a true hemisphere, but nevertheless the term allows us to visualize its growth pattern.
Physeal segment or growth plate. By cellular division, the cartilage disk increases its length interstitially and increases in diameter by apposition. Thus, the normal tendency is toward expansion in this segment. The simplest and most appropriate term for this segment is growth (Latin for physis).
Metaphyseal segment. The normal tendency in this zone is toward a reduction in shaft caliber by internal and external absorption. The term “constriction” is deeply entrenched in the literature but is not descriptively accurate. The vascular erosion and osteoclastic absorption are not constrictive but are reductive in caliber. If one were to select a new term for these processes, it would be “funnelization”. It preserves the image of these absorptive activities at the ends of the shaft, which allow for progressive narrowing of caliber and the resultant concave shape of the metaphysis as one passes from the end toward the middle of the shaft.
Diaphyseal segment. There is a tendency in the middle of the shaft to maintain a certain structural balance between the flaring, growing ends. To maintain this balance, the diameter of the diaphysis increases in width as the tubular bone grows in length. Proliferation of osteoblasts on the periosteal surface exceeds osteoclastic endosteal absorption, in that the cortex thickens and the marrow cavity widens as the tubular bone matures. The term “cylindrization” is applied to the vectors of the diaphyseal growth, which is such as to ensure a cylindrical shape to the shaft. The irregular shape of the cylinder is due to muscle pull.
3.3.1 Skeletal Growth and Modeling
To more fully understand normal skeletal growth, there are three basic considerations: (i) amplification or actual increases in size of bone, (ii) polarity or direction of growth, and (iii) time or scale of measurement. (see Fig. 4c for a depiction of the kinesis of skeletal development relative to other organ systems, and Fig. 4d for variations in polarity and amplification in various bones).
Kinesis is not a steady process and varies as a function of age. Increments in bone growth occur in three phases:
1–6 years gradual growth
6–12 years steady state
12–18 years rapid growth spurt
Amplification is the concept that the bone showing the greatest growth potential shows the greatest change because it magnifies the same defect to a greater degree.
Polarity is the concept that tubular bones grow in a differential pattern, with one end predominating over the other. The maximal direction of longitudinal growth is its polarity.
3.3.2 Classification of Bone Types
Each bone in skeletal growth is shaped or modeled as a function of its capacity for either endochondral and/or intramembranous growth depending on the anatomic location and its ultimate function as to support, protection and movement.
3.3.3 Orientation by Anatomic Region and Bone Type
There are eight anatomic regions:
Head. Skull, facial bones, and mandible
Neck. Cervical vertebrae (C1–C7), hyoid bone, and clavicle
Thorax. Ribs, thoracic vertebrae (T1–T12), sternum manubrium, and pectoral girdle
Abdomen. Lumbar vertebrae (L1–L5) and pelvic girdle (false pelvis)
Back. Vertebrae composing the spine—cervical (C4–C7), thoracic (T1–T12), lumbar (L1–L5), and sacral (S5–S1)
Pelvis. Sacrum (S1–S5), coccyx, and true pelvis
Upper limb. Pectoral girdle, arm, forearm, and hand
Lower limb. Pelvic girdle, thigh, leg, and foot.
The five types of bone shapes are long, short, irregular, flat, and special bones. Most bones are a mixture (m) of endochondral growth and intramembranous bone formation. Some are mainly endochondral (e) or intramembranous (i) bone formation.
m Long. Upper—humerus, radius, ulna; lower—femur, tibia, and fibula
e Short. Metacarpal, metatarsal, and phalanges
m Irregular. Vertebrae
m Flat. Ribs, scapula, and pelvis
i Special. Skull, face, and mandiblem
The skeletal anatomic regions can be divided into five basic bone types for the purposes of presentation. Each site is presented diagrammatically to indicate sites of maximal bone growth and modeling.
3.4 Biology
3.4.1 Molecular Mechanisms of Radiotherapy-Induced Musculoskeletal Injury
3.4.1.1 Endochondral Base and Bone
In most tissues, damage from radiation is related to both DNA damage and an apoptotic protective function regulated partially by p53. In bone, however, p53 does not usually play much of a role, even in apoptosis. In the growth plate, dividing chondrocytes are very sensitive to the damaging effects of RT. Even low doses produce significant apoptosis, resulting in cell depletion and decreased growth from that plate. The radiation of a child’s growth plate causes growth abnormalities and bone shortening described in more detail later. The exact regulators of apoptosis in the growth plate are not well described, but, oddly enough, p53 has no role in this apoptosis since it is not present in growth-plate chondrocytes (Midgley et al. 1995). Whatever the mechanism, it also affects osteocytes near the growth plate. In fact, as a response to RT, apoptosis of osteocytes occurs and increases with decreasing distance to the growth plate (Stevens et al. 2000). Osteocytes in adults, as well as osteocytes in children that are located distant from the growth plate, do not exhibit apoptosis in response to RT. In these, p53 accumulates after RT, but upstream signaling does not occur, resulting in no apoptosis (Midgley et al. 1995). Brg 1, an ATPase enzyme, may be partially responsible for this lack of apoptosis in mature-bone osteocytes, as may Bcl-2, an anti-apoptotic gene. Studies of mature-bone cells show Bcl-2 to be present in developed bone osteocytes absent from any cells in the growth plate. This absence of Bcl-2 is observational at this time, but may prove to be significant.
Since osteocytes are nonmitotic, their resistance to apoptosis is not likely to result in severe cellular damage. However, the resistance of osteocytes to apoptosis is overcome in long-term hypoxic conditions, particularly in the presence of high-dose steroids; therefore, a vascular compromise from RT may induce enough hypoxia to create necrosis of the femoral circumstances (Tsuji et al. 2006).
3.4.1.2 Striated Muscle
Undifferentiated muscle cells are as sensitive to RT damage as the chondroblasts in the growth plate, but their manifestation is different. In muscle cells, RT causes double-strand breaks (DSB) that activate DNA damage sensors, or signal transducers and effectors that determine the life or death of the cell (Latella et al. 2004). In contrast to osteocytes and chondrocytes, p53 plays an important role in muscle cells. This reaction is mediated at a checkpoint (Chk1 or Chk2) to allow either DNA damage repair or, if the damage is too severe to be safely repaired, cause the cell to undergo apoptosis. Ataxia-telangiectasia-mutated (ATM) phosphorylates Chk2 and this modification create an activating signal response to DNA damage in myoblasts. If P53 is phosphorylated at conserved residue mouse serine 18 and human serine 15 [Ser15(h)/18(m)], apoptosis is triggered (Latella et al. 2004). However, in differentiated cells, p53 is not phosphorylated; the p53 does accumulate in the differentiated cells, but it does not correspond to increased p53 transcriptional activity, so apoptosis does not occur (Latella et al. 2004). Obviously, this mechanism is displayed by terminally differentiated muscle cells to prevent cell depletion in undifferentiated muscle cells with a limited capacity to self-regenerate. Radioresistance is also present because these cells do not divide, thus DNA damage does not lead to cell death at the time of division.
Differentiated cells, the myotubes, are prone to developing late radiation fibrosis, which is volume, dose, and fraction-size dependent. Different molecular mechanisms are responsible for the two effects. Late radiation fibrosis is probably caused by the release of downstream fibrogenic cytokines such as fibroblast growth factor 2 (FGF2) and transforming growth factor beta 1 (TGFBeta1). Fibrosis is worsened by inflammation, and RT is thought to produce aberrant proinflammatory cytokines, including tumor necrosis factor alpha (TNF alpha). This cytokine causes neovascular necrosis at low doses of radiation and mature vascular necrosis at higher doses. It also helps recruit and activate macrophages into injured tissue, increasing the release of FGF2 and TGFBeta1. FGF2 attracts fibroblasts and induces mitosis, while TGFBeta1 incites fibroblast proliferation and premature end-differentiation, which leads to the extracellular matrix accumulation of glycoprotein production (Fig. 5) (Okunieff et al. 2004).
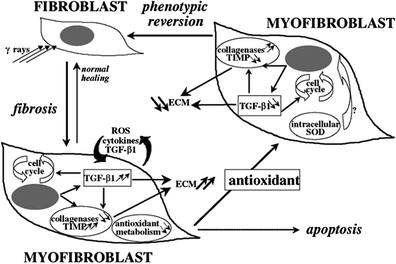

Fig. 5
Main radiation-induced fibroatrophic process (RIF) actors and possible cell phenotype reversion after antioxidant treatment. [with permissions from Delanian and Lefaix (2004)]
3.4.1.3 Joints
There is little information on the molecular cause of radiation damage to tendons and other structures of the joint.
4 Pathophysiology
4.1 Endochondral Base and Bone
The growth plate is the most sensitive structure to radiation damage. Early changes after RT include loss of chondrocytes. Near the growth plate, osteoblasts, and endothelial cells that invade the cartilage columns and produce bone exhibit pyknotic, hyperchromatic nuclei, and focal necroses (Farjardo et al. 2001). With longer followup, growth plates will show the typical pathology of mature bone, with osteoid and bone matrix replacing the chondrocytes of the immature growth plate. Thrombi from platelet-fibrin groupings can be visible in the microvasculature. There will be less new osteoid and vacant osteocytic lacunae may be visible (Farjardo 1982). This is true in mature bone as well (Fajardo et al. 2001). Years after treatment, atrophy and fibrosis of the marrow spaces will be present, replacing normal bone marrow (Fajardo et al. 2001). Eventually, fibrosis of the periosteum and endosteum will evolve. The histologic picture will be that of a cellularly depleted bony matrix. However, at times, new bone will start to form around the damaged bone (See Fig. 6a, b for the effects of radiation on the growth of different components of long bones).
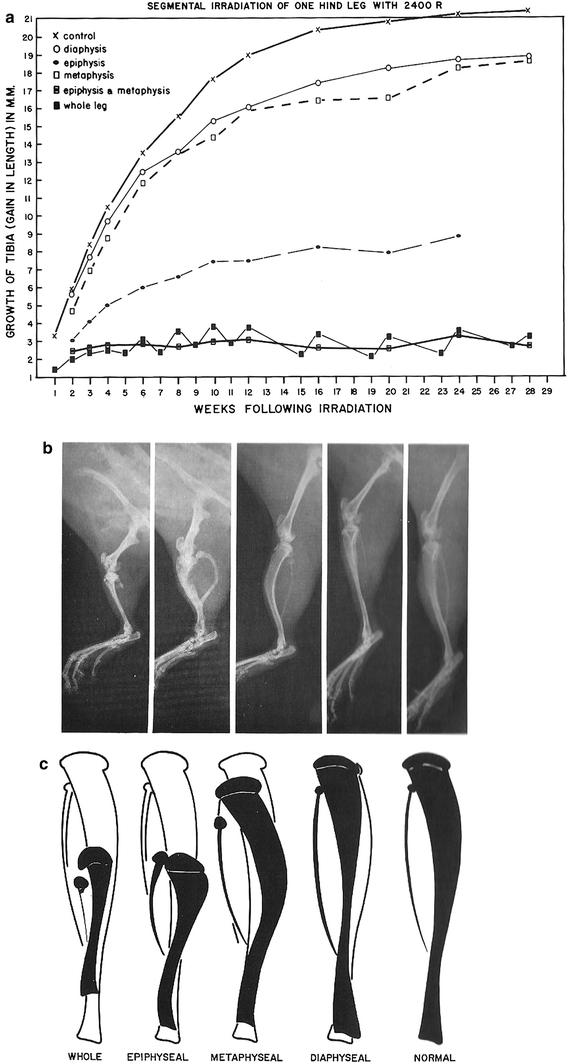

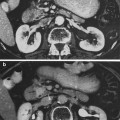
Fig. 6
a Reduced bone growth after segmental irradiation in long bone. b The radiographic correlate to (a). c is a schematic showing the impaired bone as the filled-in structure (from Rubin and Casarett 1968)
Sometimes osteoradionecrosis (ORN) results from high doses of RT. In addition to empty lacunae and pyknosis of osteocyte nuclei, Haversian vessels may show endothelial cell injury with the platelet-fibrin thrombi noted above. Demineralization of the bone by osteocytes begins at some point, evolving into a necrotic zone with a yellow–gray color seen under the microscope (Farjardo et al. 2001). Bone marrow changes may range from a hypoplastic and fatty marrow to fatty acid crystals and masses of amorphous calcium soaps (Chang et al. 1993).
The primary late effect damage to mature bone is ORN, with its accompanying loss of strength and increased risk of fracture. ORN is usually found at the site of a fracture.
4.2 Muscle
Though myotubes are nondifferentiating and resistant to genotoxic stress, stem cells and myoblasts are not. The stem cells are the most sensitive to the effects of RT. Studies on myoblasts also show significant apoptotic cell death from small doses of radiation (Olive et al. 1995). The depletion of stem cells and myoblasts from the cell pool causes significant decreases in muscle growth and size in children. Myotubes are relatively resistant to the effects of radiation. Only massive doses will produce cell death, though aggressive RT will cause late fibrosis.
The histology of radiation-induced fibrosis (RIF) varies depending on the stage of fibrosis: (a) In the initial pre–fibrotic stage, chemokines attract leukocytes to the injury site, giving the histologic appearance of chronic nonspecific inflammation (Delanian and Lefaix 2004), resulting in increased vascular permeability and edema. Collagen degradation fragments attract the local connective and epithelial tissue cells as well as blood. Necrosis of the endothelium with thrombosis can cause necrosis of the microvessels with local ischemia. Loss of this barrier probably causes connective cell exposure to foreign stimuli, triggering fibroblastic activation (Lefaix and Daburon 1998). (b) Later, in the constitutive organized phase, the injured irradiated tissue histologically appears to be primarily composed of fibroblasts and extracellular matrix (ECM). In some areas, there is a high density of myofibroblasts (activated fibroblasts) in a disorganized matrix; the rest of the tissue appears to be primarily regions of densely sclerotic ECM. (c) The constitutive organized phase evolves to a late fibroatrophic phase, during which, histologically, almost all fibroblasts are gone and the tissue is almost entirely a dense ECM. These irradiated areas are fragile and poorly vascularized, and can undergo reactivated inflammation after even a mild insult (Delanian and Lefaix 2004) (Fig. 5).
Fibrosis in muscle increases with time. The evolution of the muscle tissue from contracting, flexible myotubes to the late fibroatrophic phase, which is primarily a dense ECM with few functioning cells, produces significant function results. The muscles become firm and less pliable and lose strength and flexibility, resulting in a loss in range of motion. There can also be pain from motion, particularly in the morning with first use. If the fibrosis affects the lymphatic and vascular drainage, there can also be lymphedema with swelling and enlargement of the extremity. Note that bone deformity alters the musculature (Fig. 6b).
4.3 Joint
The most useful data on the histologic response of joint cartilage come from arthritic radiosynovectomies of animals. After injection of Re-188 intraarticular microspheres to doses of either 0.3 or 0.6 miCi, histologic specimens did not show any significant changes in the cartilage, even a year after therapy (Wang et al. 2001). However, after as little as 12 weeks, sclerosis of the subsynovium was noted after the injection of either dose (Wang et al. 2001). There is little data on the histologic response of ligaments to RT.
Radiation has been used to sterilize tendons for transplant, and has been found to reduce the tensile strength, elastic modulus, strain, and toughness of transplanted tendons. These reductions were dose dependent for strength and toughness. There was an average of 36 and 55 % loss in tensile strength at 25 and 50 kGy compared to unsterilized controls (Seto et al. 2008). This reduction is thought to be caused by reduced crosslink density and collagen fragmentation. Since these large single-fraction doses are not used in vivo, it is not clear how much of an effect fractionated RT has on the ligaments of the joint. However, it is probable that some degree of loss of strength and flexibility occurs, though it may not be clinically significant. Note that joint abnormality results from deformity in bone modeling.
4.4 Bone Dysplasia due to Radiation-Induced Errors in Modeling
In general, the severity of the radiation effect varies directly with the dose, and fractionation or protraction of the dose has been found to lessen the degree of effect. In regard to the importance of the relative radiosensitivity of osteogenic elements or processes in determining the ultimate outcome of radiation effects in growing bone, it should be emphasized that the damage to the moderately sensitive fine vasculature of the marrow, cartilage, or bone and its capability for recovery play a large role in determining the degree of recovery of bone growth processes after irradiation. Since the nondividing reserve chondroblasts of the growing physeal cartilage plate are relatively radioresistant, they can initiate the regeneration of a growing cartilage plate in the event of survival or regeneration of adequate vasculature, even after severe damage to the proliferating and growing chondroblasts and devitalization of other parts of the growth plate. This may be associated with splitting off of the devitalized portion of the plate adjacent to the metaphysis, and displacement of this separated portion of the growth plate toward the diaphysis as longitudinal bone growth is resumed and progresses.
The severity and time of appearance of, and the time and degree of recovery from, the histopathologic effect of irradiation in the zones of endochondral bone growth are dependent upon both dose and age at the time of irradiation, and so is the degree of stunting of bone length. Some of the factors responsible for this dependence are the greater radiosensitivity of the more rapidly proliferating chondroblasts associated with phases of rapid skeletal growth, and the greater potential for stunting of bone length in bones that are far from attaining their full length at the time of irradiation.
4.5 Recovery/Regeneration
4.5.1 Endochondral Base and Bone
Bone shows a high capacity for repair after damage and fracture. After the initial damage takes place, inflammatory cells infuse from blood-secreting transforming growth factor β (TGF-β) to activate MSC in the bone marrow to produce osteoprogenitor cells and ultimately osteoblasts. Initially, a soft tissue callus called procallus forms around the ends of the fractured bone and osteoblasts begin to deposit immature woven bone characterized by an irregular arrangement of collagen. Mesenchymal cells in the procallus start to form hyaline cartilage, which will undergo endochondral ossification and develop into a bony callus. Bone remodeling will eventually change woven bone into mature lamellar bone.
MSCs can also differentiate into chondrocytes, adipocytes, and stromal cells. In the past decade, there has been considerable interest in the capacities of MSCs to increase tissue repair in humans. There have been reports on the infusion of MSCs in patients with osteogenesis imperfecta (Horwitz et al. 1999). All patients had increases in total-body bone mineral content, increases in growth velocity, and reduced frequencies of bone fracture.
Another interesting area of research is osteoinductive protein therapy using bone morphogenetic proteins (BMP)-2 and -7, which have been shown to stimulate new bone formation. Despite early promising laboratory results, it has not proven useful in clinical practice, particularly after radiation damage (Wurzler et al. 1998).
4.5.2 Muscle
Skeletal muscle repair/regeneration is very limited. Adult skeletal muscle fibers and cells do not undergo mitosis. If injury happens, satellite cells residing beneath the basal lamina of skeletal muscle fibers act as precursors for a little muscle growth and repair, proliferating and fusing to form new skeletal fibers. This satellite cell pool contains a distinct population of skeletal muscle precursors (SMPs) that can function as muscle stem cells. The efficacy of myogenic stem-cell transplant for treating muscle degenerative disease and other injuries is the subject of active laboratory research (Cerletti et al. 2008).
Since RT causes significant depletion of stem cells and myoblasts from the cell pool, it substantially limits the repair capacity of muscle (Olive et al. 1995). It remains to be seen if future stem-cell research will have any impact on patients with muscular toxicities as a consequence of RT.
5 Clinical Syndromes
Developing a grading system for bone and muscle toxicity was complicated by how these organ systems encompass anatomically and functionally diverse sites, and because changes only became evident with long-term follow-up. Furthermore, it was clear that it was necessary to assess not only the effects of RT alone, but also the combined effects of RT with surgical resection, cytotoxic drugs, and other therapeutic interventions. The radiation therapy oncology group (RTOG), building upon clinical experience on toxicity, initiated an active effort to establish criteria and scoring for late effects. In early 1981, RTOG started to include late-effects monitoring in its protocols. The European Organization for Research and Treatment of Cancer (EORTC) used its own toxicity scoring. The National Cancer Institute created the common toxicity criteria (CTC) in 1990, but in this system mainly included acute toxicities (CTC 2008) (http://ctep.cancer.gov/reporting/ctc.html). One of the pivotal publications on this topic came from Eifel et al. (1995) who proposed a late-effects scoring system for bones. It was built on both laboratory evidence and clinical observations in children experiencing various forms of bone late effects. This groundbreaking work laid a foundation for the musculoskeletal scoring system later included in the RTOG Toxicity scoring as well as the subjective, objective, management, and analytic-late effects normal tissue (SOMA-LENT) systems developed as a result of the EORTC and RTOG joining its subcommittees aimed at standardizing toxic-effects criteria (LENT SOMA Tables 1995). (Tables 1, 2 and 3 LENT SOMA tables).
Table 1
Muscle/soft tissue: LENT-SOMA
Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
|---|---|---|---|---|
Subjective | ||||
Pain | Occasional and minimal | Intermittent and tolerable | Persistent and intense | Refractory and excruciating |
Function | Interferes with athletic recreation | Interferes with work | Interferes with daily activity | Complete lack |
Objective | ||||
Edema | Present/asymptomatic | Symptomatic | Secondary dysfunction | Total dysfunction |
Mobility and extremity function | Present/asymptomatic | Symptomatic | Secondary dysfunction | No mobility, frozen |
Fibrosis | Detectable | ≤20 % of muscle | >20–50 % of muscle | >50 % of muscle |
Atrophy | ≤10 % | >10–20 % | >20–50 % | >50 % |
Contraction | ≤10 % linear field | >10–30 % linear field | >30 % linear field | |
Management | ||||
Pain | Occasional nonnarcotic | Regular non-narcotic | Regular narcotic | Surgical intervention |
Edema | Compression | Medical intervention | Surgical intervention | |
Mobility and extremity | Occasional physiotherapy | Intermittent physiotherapy | Persistent physiotherapy or medical intervention | Surgical intervention |
Fibrosis | Occasional physiotherapy | Intermittent physiotherapy | Surgical intervention | |
Atrophy | Intermittent physiotherapy | Surgical intervention | ||
Muscle/soft tissue: LENT A | ||||
Analytic | ||||
MRI | Development of investigational testing suggested | |||
Table 2
Growing bone LENT-SOMA
Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
|---|---|---|---|---|
Subjective | ||||
Pain | Occasional and minimal | Intermittent and tolerable | Persistent and intense | Refractory and excruciating |
Abnormal gait | Slight | Noticeable limp | Severe limp | Unable to walk |
Disfigurement | Slight, not cosmetically significant | Mild cosmetic deformity | Moderate cosmetic deformity | Severe disfigurement |
Objective | ||||
Extremities | Mild curvature or length discrepancy <2 cm | Moderate curvature or length discrepancy 2–5 cm | Severe curvature or length discrepancy >5 cm | Epiphysiodesis, severe functional deformity |
Spine Sit/standing height | Mild disproportion | Moderate | Severe | |
Scoliosis | <5° | 5–10° | >10–20° | >20°, interfering with cardiopulmonary function |
Kyphosis/lordosis | Mild radiographic changes | Moderate accentuation | Severe accentuation | |
Femoral heads | Mild valgus/varus deformity | Moderate valgus/varus deformity | Mild slipped capital femoral epiphysis/epiphyseal widening | Severe slipped capital femoral epiphysis >60°;avascular necrosis |
Flat/facial bones | Slight changes, not cosmetically significant | Mild cosmetic deformity | Moderate cosmetic deformity | Profound hypoplasia or functional problem |
Management | ||||
Extremities | Minimal shoe lift | Moderate shoe lift | Surgical intervention | |
Scoliosis | Brace | Surgical intervention | ||
Femoral heads | Pinning | Hip replacement | ||
Flat/facial bones | Surgical intervention | |||
Analytic | ||||
Measure growth | No growth retardation | Growth retardation ≤1 percentile | Growth retardation >1 percentile | Growth arrest |
Radiograph/CT | Assessment of bone integrity | |||
Table 3
Mature bone (excluding mandible) LENT SOMA
Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
|---|---|---|---|---|
Subjective | ||||
Pain | Occasion and minimal | Intermittent and tolerable | Persistent and intense | Refractory and excruciating |
Function | Interferes with athletic recreation | Interferes with work | Interferes with daily activity | Complete fixation, necrosis |
Objective | ||||
Fracture | Partial thickness | Full thickness | ||
Mucosa soft tissue | Sequestration | |||
Skin over bone | Erythema | Ulcer | Sinus | Fistula |
Joint movement | <10 % decrease | >10–30 % decrease | >30–80 % decrease |
|