, Marilyn J. Siegel2, Tomasz Miszalski-Jamka3, 4 and Robert Pelberg1
(1)
The Christ Hospital Heart and Vascular Center of Greater Cincinnati, The Lindner Center for Research and Education, Cincinnati, OH, USA
(2)
Mallinckrodt Institute of Radiology, Washington University School of Medicine, St. Louis, Missouri, USA
(3)
Department of Clinical Radiology and Imaging Diagnostics, 4th Military Hospital, Wrocław, Poland
(4)
Center for Diagnosis Prevention and Telemedicine, John Paul II Hospital, Kraków, Poland
Abstract
The computed tomographic angiography (CTA) imaging protocol must be tailored to the suspected cardiac lesion and the type of prior surgical repair. The relevant parameters that need to be selected prior to imaging are contrast volume, contrast injection speed, the timing of the scan, slice collimation, scan length, tube voltage (kV), tube current (mA), and pitch. In addition, the imager must decide on the use of non-ECG-synchronized acquisition versus ECG synchronization (prospective or retrospective). In general, multidetector scanner with ≥64 rows is preferred for evaluation of congenital heart disease (CHD).
The computed tomographic angiography (CTA) imaging protocol must be tailored to the suspected cardiac lesion and the type of prior surgical repair. The relevant parameters that need to be selected prior to imaging are contrast volume, contrast injection speed, the timing of the scan, slice collimation, scan length, tube voltage (kV), tube current (mA), and pitch. In addition, the imager must decide on the use of non-ECG-synchronized acquisition versus ECG synchronization (prospective or retrospective). In general, multidetector scanner with ≥64 rows is preferred for evaluation of congenital heart disease (CHD).
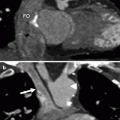
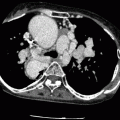
CTA protocols for assessing adult patients with known or suspected congenital heart disease are similar to standard coronary artery CTA imaging protocols with one major exception. For coronary artery imaging, scan timing is adjusted to avoid right ventricular contrast opacification since it may interfere with assessment of right coronary artery anatomy. In imaging CHD, adequate contrast opacification of both the right and left ventricles is necessary to adequately visualize the complex right- and left-sided anatomy. Not only must scan timing be altered to allow this, but at times, a delayed scan is necessary to allow adequate recirculation of contrast through complex anomalies and their potential corrections such that all the necessary anatomy is adequately opacified.
6.1 Contrast Agent Administration
Low-dose pre-contrast imaging is indicated in patients who have prior surgical repairs as it allows identification of calcified conduits and septal patches and minimizes the risk of confusing these expected changes with true postoperative complications, particularly vascular leaks. For contrast administration, a right-arm injection is preferred to avoid contrast artifacts often associated with injection into the left brachiocephalic vein. To reduce artifacts from undiluted contrast material, a saline bolus chaser should be used. We prefer the automated bolus tracking technique to determine the appropriate imaging delay. In adults, the region of interest is placed in the ascending aorta, and the attenuation threshold is set at 140 HU. An appropriate, fixed delay time (generally 15 s) may be used when the right heart, superior vena cava, or complicated shunts such as the Glenn shunt or the superior limb of a baffle are areas of interest.
To achieve both right and left ventricular opacification, a biphasic contrast injection protocol (contrast followed by a saline injection) is appropriate. Monophasic injections (contrast only) should be avoided since these cause excessive streak artifacts. If complex shunts such as Glenn or Fontan are present, additional delayed scanning should be performed 60 s after start of the contrast injection to opacify the veno-pulmonary circuit.
Imaging protocols for patients with CHD should closely mimic standard coronary artery imaging protocols since the detection of coronary artery disease remains pertinent since the prevalence of coronary artery disease is similar to that of the general population. In fact, recent studies support coronary angiography in patients with CHD older than 40 years before corrective surgery [1]. It is not unusual to perform corrective surgery in adults with congenital heart disease simultaneously with coronary artery bypass grafting [2]. In order to diagnose coronary artery images, aggressive heart rate reduction is required to prevent motion artifact. We prefer a resting heart rate of ≤60 beats per minute which not only helps to prevent motion artifact but also allows the use of radiation reduction protocols.
6.2 Slice Collimation (Slice Thickness)
Thicker detector collimation (2 mm) is preferable to reduce radiation dose and improve image quality, although visualization of small structures is diminished. In addition, the CTA acquisition time is lower when using thicker collimation, and consequently radiation dose and respiratory motion artifacts are reduced. However, if evaluation of small intracardiac structures or the coronary arteries is indicated, thinner collimation should be used.
Contrast volume is usually between 80 milliliters (ml) and 120 ml with higher volumes being used for more complex shunting anomalies to allow adequate mixing of contrast. The injection rate is generally between 5 and 7 ml/s.
6.3 Scan Length (Z-Axis Coverage)
Since the CTA radiation dose is directly proportional to the scan length, it is essential that the scan length be minimized to include only the area of interest. The typical scan length for adults is 12–13 centimeters (cm) and extends from just below the carina to slightly below the diaphragm. If great vessel anomalies are expected, a longer scan length extending superiorly may be required to allow visualization of the entire aortic arch area. A recent study has shown that for each 1 cm reduction in scan length, the retrospectively gated CTA radiation dose is reduced by 5 % [3].
6.4 ECG-Controlled Tube-Current Modulation
Modulating tube current during the R–R interval (ECG current modulation, ECG CM) has proven to be an effective dose reduction strategy in retrospective ECG-gated CTA [4]. Using ECG CM, the tube current outside the predetermined cardiac cycle is reduced relative to the current inside the pulsing window. In one series, tube current was maintained at its maximum during diastole and reduced to approximately 20 % of the maximum in systole. This approach has been shown to reduce radiation dose by as much as 40 % without loss of image quality [4]. The optimal ECG pulsing window for cardiac CTA depends on the patient’s heart rate [5, 6].
6.5 Tube Voltage
Reducing the tube voltage from 120 to 100 kV can reduce radiation dose by up to 50 % [7]. Using a kV of 80 in appropriate patients may further reduce radiation exposure [7, 8]. In patients who weigh ≤85 kg or who have a body mass index (BMI) of ≤30 kg/meter (m)2, a tube voltage of 100 kV is appropriate. Patients who weigh ≤60 kg or in those with a BMI ≤ 25 kg/m2, a tube voltage of 80 kV may be used.
6.6 Pitch
Pitch is defined as the ratio of table travel (mm) per gantry rotation to scan length. The latest generation of dual-source CTA technology permits scanning at very high pitches. In single-source CTA, the maximum pitch is limited to approximately 1.5. In the high-pitch dual-source scanner, the second detector system is used to fill the imaging gaps created by high-pitch imaging [9]. By interweaving data from the two detectors, the pitch can be increased to as high as 3.4, thus reducing the acquisition time to image the entire chest to below 1 s [10




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree