Dilation of stenoses of the major intracranial arteries is now technically possible in many cases. Using proper precautions, most procedures can be performed without complications today, but the safety margin will likely be improved with refinement of current devices and the introduction of new devices made specifically for this indication. Early experience with these techniques is promising for lowering the risk for recurrent ischemic events in patients who have symptomatic intracranial arterial stenosis refractory to medical therapy. This article describes the steps taken to perform transluminal balloon angioplasty and stent-assisted angioplasty for intracranial atherosclerotic stenosis, from patient preparation through follow-up, including procedural steps and device selection.
Patient selection
Elsewhere in this issue, the benefits and risks associated with endovascular treatment of intracranial atherosclerotic stenosis are weighed. In general, a patient selected for this procedure is one who has symptoms of transient ischemic attack (TIA) or stroke related to the target lesion, who has failed a trial of antiplatelet or anticoagulant therapy, and who has a stenosis that can be reached and dilated with reasonable safety using currently available devices. Stenoses of the larger arteries, such as the distal internal carotid artery, distal vertebral artery, basilar artery, and proximal middle cerebral artery, typically are dilated, rather than those of the smaller or more distal vessels. Although MR angiography or CT angiography screening examinations may identify an intracranial stenosis, conventional angiographic assessment of the stenosis should be performed at some point before treatment, not only to define the target lesion better, but to assess further the route to be taken to reach the stenosis with catheters and devices. The findings of the conventional angiogram, MR or CT imaging studies, and the history and clinical examination can be presented and discussed at a multidisciplinary conference to arrive at a consensus recommendation for endovascular treatment, continued medical management, or surgery, such as an arterial bypass, in select cases.
Timing of the procedure
For a patient having infrequent, related TIAs, a procedure to dilate a symptomatic intracranial arterial stenosis can be performed electively, when antiplatelet pretreatment is optimized and warfarin, if prescribed, has been discontinued far enough in advance so as to be ineffective at the time of the procedure. For a patient having frequent or “crescendo” TIAs, the procedure can be performed urgently. In such a situation, antiplatelet drugs can be given with loading doses (see later discussion) if a patient has not been maintained on those drugs in the week before the procedure. Warfarin can be reversed by intravenous administration of fresh frozen plasma, for an urgent procedure.
For a patient with a related acute stroke who is stable or improving following initial presentation, the procedure can be scheduled after a 4- to 6-week period of medical therapy to reduce the risk of hemorrhagic transformation of the infarction or hemorrhage related to a perfusion pressure breakthrough phenomenon. For a patient with an acute stroke who is continuing to decline as a result of extension of the infarction or rethrombosis at a site of stenosis, dilation of the stenosis is sometimes performed emergently as an adjunct to intra-arterial thrombolysis within the time frame observed for use of lytic drugs in acute stroke (usually within 6 hours of symptom onset in the carotid circulation, or longer in the case of basilar thrombosis).
Timing of the procedure
For a patient having infrequent, related TIAs, a procedure to dilate a symptomatic intracranial arterial stenosis can be performed electively, when antiplatelet pretreatment is optimized and warfarin, if prescribed, has been discontinued far enough in advance so as to be ineffective at the time of the procedure. For a patient having frequent or “crescendo” TIAs, the procedure can be performed urgently. In such a situation, antiplatelet drugs can be given with loading doses (see later discussion) if a patient has not been maintained on those drugs in the week before the procedure. Warfarin can be reversed by intravenous administration of fresh frozen plasma, for an urgent procedure.
For a patient with a related acute stroke who is stable or improving following initial presentation, the procedure can be scheduled after a 4- to 6-week period of medical therapy to reduce the risk of hemorrhagic transformation of the infarction or hemorrhage related to a perfusion pressure breakthrough phenomenon. For a patient with an acute stroke who is continuing to decline as a result of extension of the infarction or rethrombosis at a site of stenosis, dilation of the stenosis is sometimes performed emergently as an adjunct to intra-arterial thrombolysis within the time frame observed for use of lytic drugs in acute stroke (usually within 6 hours of symptom onset in the carotid circulation, or longer in the case of basilar thrombosis).
Patient preparation
An elective patient is usually pretreated (assuming no aspirin allergy) with a combination of aspirin and a second antiplatelet drug to lower the risk of a thromboembolic complication during or after the procedure. Aspirin reaches full effectiveness in most patients within 1 to 2 hours of an oral dose, so timing of the initiation of aspirin pretreatment is not as critical as for the second drug. Aspirin is usually given as 325 mg orally per day. Clopidogrel is the most commonly used and preferred second antiplatelet drug, based on experience from coronary interventions. Clopidogrel, given as 75 mg orally per day, the routine maintenance dose, should be started 5 days in advance of the procedure to reach full effectiveness. A common pretreatment regimen for elective patients is thus, aspirin, 325 mg, and clopidogrel, 75 mg, orally per day beginning 5 days before treatment and including the morning of treatment.
For patients allergic to aspirin, aspirin is omitted. For patients allergic to clopidogrel, other options exist. Cilostazol, 100 mg orally twice daily (taken on an empty stomach), can be substituted for clopidogrel. A combination of aspirin, 25 mg, and dipyridamole, 200 mg, one capsule orally twice daily, can be also substituted for clopidogrel and the additional aspirin dose decreased from 325 to 81 mg daily. Headache and gastrointestinal side effects are more common with cilostazol and dipyridamole than with clopidogrel. Ticlopidine, 250 mg orally twice daily (taken with food), is another substitute for clopidogrel, but patients must be periodically checked for thrombocytopenia as a side effect of the drug.
For urgent procedures, a routine 325-mg dose of aspirin is given before the procedure, and clopidogrel is given with a loading dose of 300 to 450 mg, administered at least 6 hours before the procedure, if possible. For emergent procedures, intravenous antiplatelet agents can be given in place of the oral antiplatelet agents at considerably greater cost. Abciximab for central nervous system (CNS) use can be given as a 0.20 mg/kg IV bolus immediately before the intervention, with the maintenance IV drip of 0.125 mcg/kg/min (maximum 10 mcg/min) for 12 hours as an option during and after the procedure. Alternatively, eptifibatide can be given as two IV boluses of 180 mcg/kg (maximum 22.6 mg) administered 10 minutes apart, with the maintenance IV drip of 2 mcg/kg/min (maximum 15 mg/hr) for 12 hours as an option during and after treatment.
Warfarin is usually discontinued 3 to 4 days before an elective procedure to allow the international normalized ratio (INR) to fall into a normal range at the time of treatment. For patients judged to be dependent on systemic anticoagulation (eg, for those in atrial fibrillation with left atrial enlargement at risk for cardiogenic embolism), heparin anticoagulation can be initiated when the INR falls below a therapeutic range. For urgent or emergent procedures, fresh-frozen plasma can be given to reverse the effects of warfarin.
Anesthesia
Although angioplasty and stent deployment in the thoracic and cervical segments of the carotid and vertebral arteries are performed commonly with moderate procedural sedation, dilation of more fragile intracranial arteries is performed best under general anesthesia. Not only does general anesthesia allow optimal imaging and sizing of a stenosis, it allows stable road mapping, proper wire navigation, and accurate device positioning ( Fig. 1 ). Pharmacologic paralysis prevents patient motion from occurring at critical times in the procedure, such as during the slow, gentle dilation step, when a temporary reduction in cerebral perfusion might lead to confusion or agitation on the part of the patient. Perforation or rupture of an intracranial artery during dilation could result in a neurologically catastrophic or fatal complication, and the chance of this perforation or rupture occurring is minimized by removing patient motion from the equation with general anesthesia.
Another advantage of general anesthesia is better control of systemic blood pressure during, and immediately after, arterial dilation. Certain patients depend on pial collateral flow to maintain adequate cerebral perfusion in the territory distal to a stenosis, and, with impaired autoregulation, may depend on mildly elevated systemic mean arterial pressures to feed the pial collateral network adequately. Before the dilation of a stenosis in such a situation, it is desirable to maintain mean arterial pressures at the patient’s baseline. After a successful dilation, in the setting of impaired autoregulation, it is desirable to lower the mean arterial pressure into a normotensive range to avoid potential perfusion pressure breakthrough effects, particularly if the patient has had a recent infarction in the territory of the stenosis. The anesthesiologist can begin intravenous infusions of drugs such as phenylephrine to raise pressure if necessary after induction, or nicarpidine to lower pressure after dilation, for prolonged effects, or adjust delivery rates of anesthetic agents, or administer other drugs for short-term effects on systemic pressures.
Access
The standard femoral arterial approach works well in most cases, but arterial anatomy defined by the aortic arch injection and catheterization experience from the diagnostic cerebral angiogram may indicate that an alternative access approach would work better. If it is difficult to place a diagnostic cerebral catheter in an artery from a femoral approach, it will be even more difficult to advance a relatively stiff stent delivery balloon catheter against the resistance of vessel tortuosity to achieve success in distal navigation and delivery through that artery. Choice of access site is a concern for target stenoses in the posterior circulation more often than for those in the anterior circulation in actual practice.
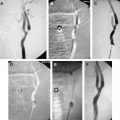
If the target stenosis is in a distal vertebral artery, close attention should be paid to the anatomy of the proximal vertebral artery to decide on the access approach. In the special situation in which the vertebral artery arises from the aorta directly and is of too small a diameter to accept a guide, a femoral approach would still be taken, but the guiding catheter or sheath chosen would need to be one that offered the best possible stability in the aortic arch. In the situation in which the origin of the vertebral artery from the subclavian artery is more parallel to the distal subclavian than to the proximal subclavian artery, a brachial approach would offer better stability and would be preferable to a femoral approach ( Fig. 2 ). A brachial approach might also be better for situations in which the aorta is ectatic and tortuous or in which the proximal subclavian artery is diseased, even if the vertebral origin is perpendicular to the subclavian curve, because the length of the guiding catheter in the body is reduced when a brachial approach is used. If the target stenosis is in the basilar artery, the approach taken to reach the stenosis is usually through the dominant vertebral artery, unless that distal vertebral artery is more diseased than the nondominant vertebral artery, and the access approach chosen, femoral or brachial, should be one that is optimal for catheterizing that proximal vertebral artery.
Sometimes, extreme tortuosity of the brachiocephalic arteries hinders catheterization of the cervical carotid artery. If the target stenosis is in the carotid territory and placement of a stable guiding catheter or sheath is impossible from a femoral approach, a brachial approach to carotid catheterization and an exchange for a guiding catheter can be attempted, as can a direct cervical common carotid artery puncture for placement of a short arterial sheath. Using a direct carotid approach leads to the issue of achieving hemostasis after removal of the sheath. Compressing a carotid artery in which a stent has just been placed distally risks diminishing distal flow and local hematoma development, particularly if the patient remains heparin-anticoagulated, so an alternative to compression is having a neurosurgeon or vascular surgeon remove the sheath and oversew the arterial defect after the procedure.
Guiding catheters and sheaths
One of the most important aspects of a successful procedure is establishing a stable catheterization of the parent artery. It is assumed that the reader has knowledge of, and experience with, catheterizing common carotid and vertebral arteries using standard cerebral catheters and guidewires.
For a target stenosis in a distal internal carotid or proximal middle cerebral artery to be treated from a femoral approach, the ideal platform is a firm guiding catheter or sheath with a soft and flexible tip that is positioned in the upper cervical internal carotid artery. Softer guide catheters (eg, 5 French Envoy, Cordis, Miami, FL) typically used for other interventional neuroradiologic procedures for placement of very flexible and trackable microcatheters may not provide the degree of support necessary for delivery of less flexible balloon or stent delivery catheters. Such small-diameter, soft guide catheters may be pushed back into the aorta by forward force applied to relatively stiff device catheters within them. More suitable guides for intracranial angioplasty or stent delivery are slightly larger, with sturdier walls (eg, 6 French Guider, Target Therapeutics/Boston Scientific, Fremont, CA, for less tortuous routes, or the 80-cm long 6 French Shuttle sheath, Cook, Inc., Bloominton, IN). Even more support is sometimes necessary, and in those cases, a 6F guiding catheter can be placed coaxially within a 6F guiding sheath. One should always check the minimum inner diameter guiding catheter required for the intended stent delivery system and be certain the guiding catheter chosen has an inner diameter large enough to be compatible with it.
Placement of the guiding catheter or sheath in a carotid artery is usually performed by exchanging the diagnostic catheter over an exchange wire for the guide. In most situations, this exchange can be performed with the diagnostic catheter and exchange wire positioned in the upper cervical common carotid artery, to avoid inducing vasospasm in the internal carotid artery. Most often, a standard 3-mm J exchange wire will suffice, but in cases in which tortuosity might interfere with the catheter exchange in the common carotid artery and more wire support is needed, the diagnostic catheter can be advanced into the external carotid artery and the exchange wire can be advanced out an external carotid branch. In such cases, an exchange wire with a flexible hydrophilic tip and firmer principal wire (eg, Connors, Cook, Inc.) is useful. If an arterial sheath has been placed in the femoral artery during initial access, it will need to be of a sufficient size to allow passage of the chosen guide catheter (eg, 6F for a 6F guiding catheter), but will need to be removed over the exchange wire along with the diagnostic catheter if a guiding sheath (eg, 6 French Shuttle) is chosen for placement in the parent artery. Once the guiding catheter or sheath is delivered over the exchange wire into the distal common carotid artery, it can be advanced over a hydrophilic wire (eg, Roadrunner, Cook, Inc.) into the cervical internal carotid using a road map. Advancing the guide or sheath as far as possible into the cervical internal carotid artery will offer the best support, but care should be taken not to advance any portion of a relatively stiff sheath, its inner dilator, or a guiding catheter into the petrous internal carotid artery, to avoid dissecting the vessel. If the proximal internal carotid artery is too sharply angled relative to the common carotid artery (eg, at a 90° angle to each other), or if there is atherosclerotic stenosis of any significant degree at the internal carotid artery origin, the guiding sheath or catheter is left in the distal common carotid artery.
For a target stenosis in the distal vertebral artery or basilar artery, the caliber of the vertebral artery, the origin of the vertebral artery, and the access site chosen will influence the choice of guiding catheter and placement steps. A small-to-medium–diameter vertebral artery may limit the guiding catheter to a 5F size (eg, Envoy), whereas a 6F guide catheter can be used in a large vertebral artery. A larger, stiffer guiding sheath (eg, 6 French Shuttle) generally is not used in a vertebral artery proper, but may be very appropriate if the guiding catheter tip must be positioned in the subclavian artery or aortic arch just proximal to the vertebral artery origin. Guiding catheters larger than 5F generally are not necessary or used from a brachial approach.
Placing a guide catheter in a vertebral artery from a femoral approach is often done as an exchange for a diagnostic catheter over an exchange wire, similar to the steps described earlier for placement of a carotid guide. The exchange wire in the smaller vertebral artery is generally a straight-tipped or 1-mm J-tipped wire, rather than the 3-mm J-tipped wire. From a brachial approach, or from a femoral approach with very little tortuosity, the guide catheter can be placed primarily over an angled wire. A good combination for primary guide catheter placement, for example, is a 90-cm–long 5F Envoy catheter in which a 100-cm–long 4F Cordis H1 catheter has been placed coaxially hub to hub, directed over a Cook 0.035-in Roadrunner wire, advanced using a road map for optimal positioning. The 4F inner catheter tip protrudes a few millimeters outside the guide catheter tip, is used to help direct the wire and guide catheter into the vertebral artery origin, and serves to smooth the transition from the diameter of the wire to the guide catheter in this more fragile artery. (Note that the 4.1F Cook diagnostic catheters will not fit coaxially within the 5F Envoy catheters.) As in the carotid artery, the further the guide catheter can be advanced into the cervical vertebral artery, the more stable the system will be, but kinks, turns, or stenoses in the proximal vertebral artery may limit advancement. The guide catheter should not be forced through acutely angled vessel segments, or further superiorly than the horizontal turn of the vertebral artery at C2, to avoid dissecting the vessel or inducing vasospasm that would obstruct flow. Once the guide catheter is positioned, any coaxial catheter is removed. (Because there is no flush around the 4F catheter within the 5F guide, the catheterization with that coaxial system should be performed gently but without delay, and the guide catheter should be aspirated and flushed, as would be the case after any guide catheter placement.)
A test injection of contrast into the guiding catheter or sheath following final positioning should be performed to be certain the maneuvers to place it did not induce significant vasospasm in the internal carotid artery or vertebral artery before proceeding. If significant vasospasm is seen, it may be necessary to withdraw the guide slightly, wait several minutes until the vasospasm resolves, or treat the vasospasm with intra-arterial drugs (eg, papaverine, 1–10 mL of a 3 mg/mL solution, or verapamil, 1–10 mL of a 1 mg/mL solution, in normal saline given at 1 mL/min or slower, so as not to lower systemic arterial pressure).
Baseline angiography
Initial angiography performed during the procedure, after placement of a diagnostic cerebral catheter, guiding catheter, or sheath in the parent artery, is performed to define the stenosis, to measure the diameter of the artery accurately at the stenosis and in nondiseased arterial segments immediately proximal and distal to the stenosis, and to measure the length of the stenosis to be treated. The arterial tree distal to the stenosis is imaged in standard projections to compare distal branches before and after treatment of the stenosis, to identify any emboli that might result, and the stenosis is examined in a sufficient number of projections to define optimal working projections for navigation of a balloon or stent delivery catheter and its wire. If the angiographic unit does not have a built-in calibration and vessel-measurement system, reference markers may be placed on the head for calibration of the angiographic measurements. Some wires have calibration markers that can also be used to measure the length of a stenosis if placed in the field of view. As a final check on the validity of the measurements, one should be aware that the diameter of the average internal carotid artery is approximately 5 mm in the petrous segment and approximately 4 mm in the supraclinoid segment, the diameter of the average proximal middle cerebral artery (the M1 segment) is approximately 2.7 to 3.0 mm, and the diameter of the average basilar artery is approximately 3 mm.
Anticoagulation
Systemic anticoagulation can be initiated following placement of the arterial sheath or guide catheter, but should be in effect for navigation of the wire through the stenosis and delivery of the dilating device. Intravenous heparin dosing can be weight-based or, better, based on activated clotting time (ACT) measurements. If weight-based, heparin can be given as an intravenous bolus of 70 to 100 units per kilogram of body weight. If ACT-based, a baseline ACT is obtained, and heparin is administered intravenously until the ACT is at least double the baseline value, or 300 to 350 seconds. Because these procedures are not usually lengthy, continuous heparin infusions after the initial dose or doses usually are not required, but if used, continuous infusions are begun at 7 to 10 units/kg/hour and adjusted by serial ACT measurements to maintain the ACT at 300 to 350 seconds.
In the special circumstance in which a IIb/IIIa platelet inhibitor is used during a procedure, either as a substitute for oral antiplatelet medication or to treat a thromboembolic complication, the heparin dosing is adjusted or partially reversed to achieve an ACT of approximately 160 seconds, to reduce the risk of a hemorrhagic complication through an additive effect of the two drugs. Administration of intravenous protamine sulfate at a dose of 10 mg for each 1000 units of heparin to be reversed is effective for this purpose, with the maximum recommended protamine dose being 80 mg. Protamine is injected slowly over 2 to 5 minutes to avoid a hypotensive reaction.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree