Carotid angioplasty and stenting (CAS) is an alternative technique to restore a normal lumen for patients who are at high risk for adverse effects with carotid endarterectomy (CEA). CAS has been shown to be of benefit to several groups of patients who have carotid disease and who ordinarily are excluded from many CEA trials. Government payors have approved embolic protection devices (EPDs) and stents for the now reimbursable procedure. In fact, the Centers for Medicare and Medicaid Services are now mandating use of the EPDs in CAS to issue payment. The prudent practitioner will carefully select the patients for CAS with EPD and CEA, so that patients will have the safest opportunity to avoid the devastating effects of cerebrovascular accidents.
In the article by Loh and Duckwiler, the authors presented the rationale for selecting patients for carotid artery revascularization. They discussed the indications for the procedure and the ensuing benefits, and mentioned the techniques of carotid endarterectomy (CEA) and carotid angioplasty and stenting (CAS). In this article, the authors discuss the decision process leading to choices in the materials and techniques used in CAS, the procedure itself, and the results. The goal of CAS is restoration of a near-normal lumen. The angioplasty expands the lumen in the diseased stenotic carotid artery, and the stent prevents recoil and restrains protruding intima and plaque, thereby maintaining the just-restored lumen.
Preparation
One of the most important steps for any successful procedure is preparation. For CAS, the clinical and imaging evaluation of the patient to determine the suitability of the procedure is essential. The symptomatology determines whether a patient will be low or high risk, or is perhaps not even a candidate for CAS.
The patient’s imaging must be reviewed to evaluate both the vasculature and the intracranial contents. If the patient is symptomatic, it is important to determine that the symptoms are caused by ischemia, rather than something else. A recent CT or MR image will detect an infarction or hemorrhage while excluding other processes such as a tumor. Evidence of an infarction may also determine the timing of the procedure. Large areas of damage may lead to hemorrhagic conversion when the supplying carotid artery patency is restored. If the patient has not recovered significantly, or if the infarct volume by imaging is more than one third the territory of the stenotic carotid distribution, the authors usually perform angioplasty and stenting 1 month after the incident. This waiting period seems to allow the injured vasculature and brain to heal sufficiently to lessen the risks of hemorrhage after revascularization. The risk of this delay is that the patient may have further ischemic events despite antiplatelet agents. In fact, the tightly stenotic carotid artery may even occlude during the waiting period. In the NASCET study, the nearly occluded (“string sign”) symptomatic patients seemed to do reasonably well with medical therapy, with a 1.7% cerebrovascular accident (CVA) rate at 1 month that increased to 11.1% at 1 year, and those undergoing CEA had a stroke rate of 6.3% . Of course, as yet no data exist on angioplasty and stenting in these patients.
During the clinical evaluation, the interventionalist can determine the patient’s ability to cooperate. Most procedures are performed with conscious sedation, but some patients may have difficulty cooperating when sedated. In addition, the patient must be able to sustain an open airway while supine under normal (unchallenged) circumstances. Patients who have a history of a contrast allergy should be premedicated with prednisone, 50 mg, by mouth every 6 hours for three doses ending 1 hour before the procedure; just before the procedure, diphenhydramine, 25 mg IV, and an H2 blocker such as cimetidine, 300 mg IV, will prevent and lessen adverse contrast reactions in allergic patients. In cases of severe contrast reactions, intubation for airway control can prevent the respiratory effects of laryngospasm or bronchospasm. Assessment of the patient’s renal function will also assist in the selection of an appropriate contrast agent (non-ionic isosmolar) (Visipaque, Amersham PLC, Little Chalfont, Buckinghamshire, UK) for those at risk for the development or worsening of renal failure. In these circumstances, administration of a bicarbonate drip (3 amps bicarbonate in 1 L normal saline at 3 mL/kg for 1 hour before the procedure and 1 mL/kg during the procedure and for 6 hours afterwards) may lessen the incidence of postprocedural renal failure. In addition, the administration of n-acetylcysteine, 600 mg by mouth twice daily the day before and the day of the procedure, may be helpful.
Antiplatelet agents are initiated 5 days before elective procedures: 325 mg of aspirin and 75 mg of clopidogrel bisulphate daily. If the patient has gastrointestinal upset, enteric-coated aspirin can be substituted. For more urgent situations, the patient can be loaded with 300 to 450 mg of clopidogrel bisulphate several hours before the procedure. Intravenous IIb/IIIa inhibitors seem to increase the risk of intracranial hemorrhage and are not recommended for routine CAS .
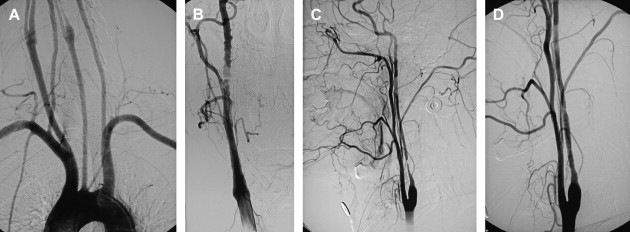
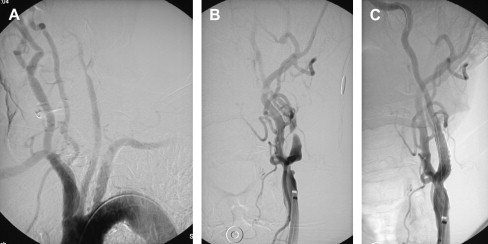
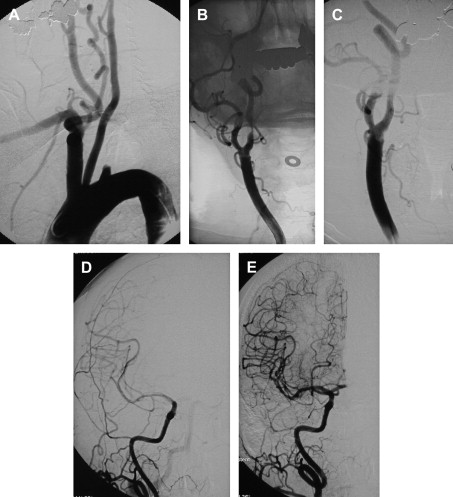
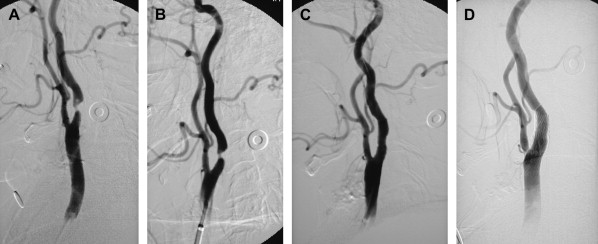
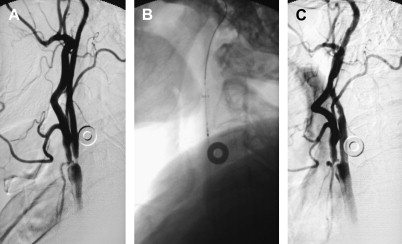
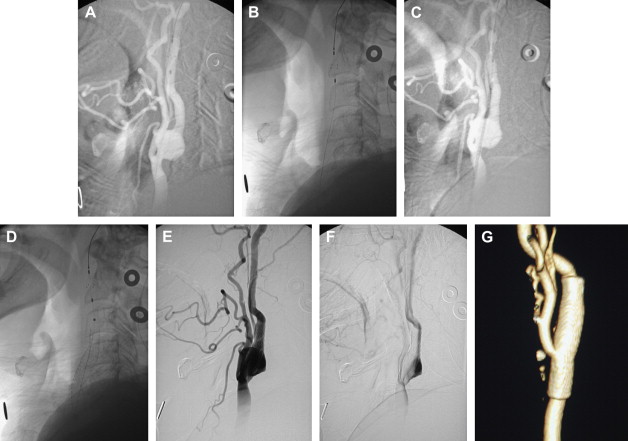
If an arteriogram was performed previously, review of it will demonstrate the aortic arch, the target carotid artery in the neck, and the blood supply to the ipsilateral cerebral hemisphere. The degree of elongation of the aortic arch will help to determine the likelihood of success of a transfemoral approach or the need for an alternative transbrachial or transradial puncture. If the line of origin of the brachiocephalic vessels arises parallel to the upper convexity of the aortic arch (type I aortic arch), the desired carotid artery may be selected using the transfemoral approach with almost no difficulty ( Fig. 1 ). Similarly, if the brachiocephalic vessels arise from the aortic arch between the outer and inner convexities of the aortic arch (type II aortic arch), selecting the desired vessel will be only slightly more difficult ( Fig. 2 ). It is only with the type III aortic arch, where the innominate artery arises below the level of the inner convexity of the aorta, that catheterizing the innominate artery, right common carotid artery, or left common carotid arteries is difficult ( Fig. 3 ). In this instance, it may be difficult to exchange the diagnostic catheter for a guiding catheter or long sheath. Origin of the left common carotid artery from the innominate artery (the so—called “bovine arch”) may cause problems for selective catheterization, similar to the type III aortic arch. In these two situations, the transbrachial or transradial approach may be appropriate. (All the authors’ CAS procedures have been performed transfemorally.) The direct carotid approach should be used only in patients who are non-CEA candidates and have high lesions and no other access. In these patients, the access site is closed operatively so that the carotid artery is not compressed in an attempt at hemostasis. Successful hemostasis is essential with the direct carotid approach because hematoma formation may compromise the airway. Finally, the carotid artery itself is evaluated because tortuosity of the common carotid artery may cause problems for catheterization. Tortuosity of the internal carotid artery may alter the choice and placement of an embolic protection device (EPD) and the type and site of the stent to be deployed, and even preclude performance of the procedure itself if there are extreme elongation and loops in the carotid artery at or near the treatment site (see Fig. 3 ). In these circumstances, CEA may be the more prudent choice. Other anatomic CAS exclusionary criteria include thrombus at the treatment site ( Fig. 4 ), long subtotal occlusion (string sign), and, of course, total occlusion.
Calcification at the angioplasty site can be problematic. Calcified plaque resists expansion and if it responds to the angioplasty, it is by fracturing, rather than by stretching or compressing. Expansion of the lumen occurs by stretching of the less involved (more normal) carotid wall. The goal of angioplasty when there is heavily calcified plaque at the angioplasty site is a reduction of the stenosis to less than 50%. Even if dilation to a more normal lumen has somehow been achieved with an aggressive angioplasty, this lumen will not be maintained by the stent because of the recoil of the residual artery wall. Attempts at overcoming the resistance of the heavily calcified arterial wall may lead to arterial rupture .
During the review of the patient’s arteriogram, the status of the opposite carotid artery and any vertebral collaterals is also informative and is particularly important if there are significant stenoses or occlusions and the operator is contemplating using flow reversal with the Parodi anti-embolism system. Although the patient may tolerate cessation or reversal of flow for short periods during the procedure, the risks could be higher than with the filter-type devices.
If the patient’s previous evaluation has been with Doppler ultrasound, MR angiography, or CT angiography, a formal arteriogram is performed immediately before the contemplated angioplasty and stenting. In most instances, the arteriogram is performed after placement of a 5F sheath in one of the femoral arteries. The 5F catheters are used for the arch aortography and the selective diagnostic arteriograms, which consist of an aortic arch, bilateral common carotid injections in the neck and head, and at least one vertebral artery (see Fig. 1 ). Evaluation of the intracranial circulation excludes more significant intracranial stenosis than the target carotid artery. Thus, the appropriateness of the proposed intervention and the suitability of the accompanying vasculature and planned access routes can all be determined before the procedure.
Embolic protection devices
EPDs have been approved recently for use with CAS. The theory behind their design is reasonable because most CVAs and transient ischemic attacks (TIAs) during CAS are caused by emboli. Whether the emboli are due to thrombus formation on the various catheters and devices, platelet aggregation, or liberated atheromatous debris does not matter because the effect is the same, obstruction of the cranial arterial vessels. Only rarely are procedural ischemic events related to hypoperfusion, which is usually due to hypotension and bradycardia. Therefore, the concept of embolic protection is reasonable.
Currently, the four approved EPD systems are all filters with pores that allow blood to pass, but capture emboli or debris. They are mounted on a guidewire, the tip of which must first cross the stenosis; then, the restrained filter crosses the area. This procedure requires a residual lumen of approximately 2 mm. If the lumen is smaller, an unprotected angioplasty with a small balloon must be performed to allow passage of the guidewire and filter. In both instances, risks include obstruction of the lumen, dislodgement of debris (emboli), and dissection. The EPD wires and filters are not as readily maneuverable as standard .014- or .018-in guidewires. Moreover, the filter may not trap all the emboli because of imperfect apposition to the walls of the carotid. It may also become clogged with debris, which significantly obstructs flow. The four FDA-approved EPDs approved by the Food and Drug Administration (FDA) for CAS are available in varying sizes, depending on the size of the artery in which they are to be placed. They should be slightly larger than the internal carotid artery to assure wall-filter apposition. The first approved EPD was the Accunet, initially developed by Guidant (St. Paul, MN), which has been sold to Abbott (Abbott Park, North Chicago, IL) ( Fig. 5 ). Abbott also has an EPD named the Emboshield. Recently, approval for the Angioguard (Cordis Neurovascular, Miami Lakes, FL) ( Fig. 6 ) and the Spider (eV3, Irvine, CA) has been granted. A similar filter device approved for use in the heart but not for the carotid artery is the FilterWire EZ from Boston Scientific ( Fig. 7 ).
Another not-yet-approved distal EPD is a microballoon, which is placed across the stenosis and inflated. The advantage is complete obstruction to distal embolization. The disadvantages are the resultant complete obstruction to internal carotid artery flow, which is not tolerated in about 5% of patients, and the need to cross the stenosis.
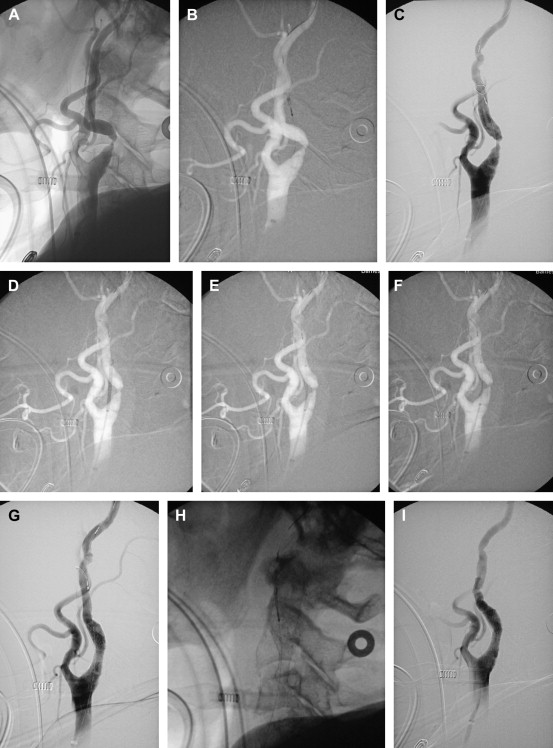
An entirely different concept that is not yet approved for use in the carotid artery is the Parodi anti-embolism system. With this device, the technique is changed; the lesion is not crossed until the EPD is in place ( Fig. 8 ). A large-bore guiding catheter with a balloon at its tip is placed in the common carotid artery. Through the guiding catheter lumen, a second, smaller balloon is positioned in the external carotid. Femoral venous access is also obtained. After the angioplasty device is ready to be positioned, the balloon in the common carotid artery is inflated, thereby obstructing flow. The balloon in the external carotid artery is also inflated. The balloon guide catheter then becomes a conduit for reversed flow from the opposite carotid artery or vertebral basilar system. This flow is directed through a filter and returned to the patient through the femoral vein. A standard angioplasty and stent procedure is then performed. At the conclusion of the CAS procedure, the balloon in the external carotid artery is deflated and removed. The guide catheter balloon is then deflated. The major advantage to this system is that the lesion does not have to be crossed without the protection in place. The major disadvantage is that not all patients have sufficient collateral supply to tolerate the occlusion, and a minor disadvantage is the larger-diameter 10F sheath necessary for insertion of the balloon guide catheter.
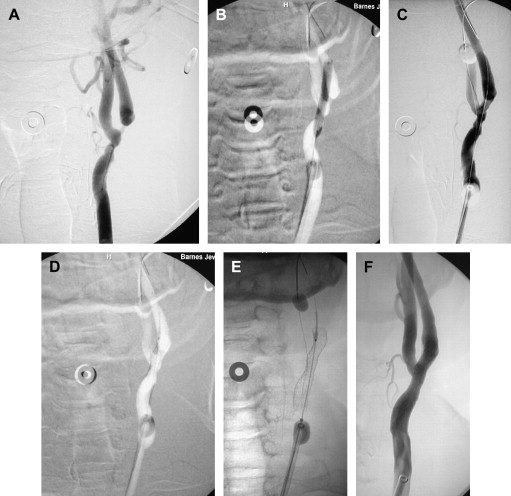
The authors have used EPDs for the past year and a half and found that the complication rate is slightly higher because of the learning curve with each EPD.
Although EPDs make intellectual sense, it is unclear whether their use results in a reduction in TIAs, CVAs, and death. Postulated reasons for untoward events despite EPDs include the necessity of crossing the lesion with the filter; movement of the guidewire and filter during the procedure, which may lead to spasm or intimal damage; failure to capture some of the emboli because of incomplete wall apposition; and overwhelming of the filter with debris. Operator inexperience and unfamiliarity with the device may also lead to complications.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree