Temporal Bone
The temporal bone is divided into five parts, which are subsequently described. The squamous portion (1) is anterolateral, forming the upper part of the temporal bone. It is thin and shell-like, and forms the lateral wall of the middle cranial fossa. The temporalis muscle attaches to the squamous portion of the temporal bone. The zygomatic process arises from the lower portion and arches anteriorly, with the masseter muscle originating from its medial surface. The mastoid portion (2) contains the antrum (a large central mastoid air cell) which communicates posteriorly with the smaller mastoid air cells and anteriorly with the epitympanum (attic) via a small canal (the aditus ad antrum). The petrous portion (3) lies at the base of the skull between the sphenoid bone anteriorly and the occipital bone posteriorly. The petrous portion contains the internal auditory canal (IAC), with its opening (the porus acusticus), and the membranous and bony labyrinths of the inner ear. The IAC is divided into upper and lower compartments by a bony crest, the crista falciformis. The upper compartment contains the facial nerve (CN VII) anteriorly and the superior vestibular division of CN VIII posteriorly. The lower compartment contains the cochlear division of CN VIII anteriorly and the inferior vestibular division of CN VIII posteriorly. The jugular foramen is bordered by the petrous temporal bone anteriorly and the occipital bone posteriorly. The jugular foramen has two compartments, the smaller pars nervosa (anteromedial) which contains the inferior petrosal sinus and the glossopharyngeal nerve (CN IX), and the larger pars vascularis (posterolateral) which contains the internal jugular vein, the vagus nerve (CN X), and the spinal accessory nerve (CN XI). The tympanic part of the temporal bone (4) is a small curved plate surrounding the external auditory canal (EAC). The styloid process (5) projects down and anteriorly from the undersurface of the temporal bone, just anterior to the stylomastoid foramen.
The middle ear (tympanic cavity) is air-filled (via the eustachian tube from the nasopharynx) and traversed by the auditory ossicles (which connect the lateral and medial walls). The tympanic membrane (TM) separates the tympanic cavity from the EAC. There are three parts: the epitympanum, mesotympanum, and hypotympanum. The superior limit of the epitympanum (attic) is the tegmen tympani (which separates the epitympanum from the middle cranial fossa) and the inferior margin is defined by a plane between the scutum (junction of lateral attic wall and roof of the EAC) and the tympanic segment of CN VII. Thus, it lies above the attachment of the TM. The lateral epitympanic recess, also known as Prussak space, is the classic location of acquired cholesteatomas. The head and body of the malleus and the short process of the incus lie in the epitympanum. The mesotympanum lies below the epitympanum, directly medial to the TM, separated from the hypotympanum below which lies inferior to the TM attachment. The mesotympanum contains the manubrium of the malleus, long process of the incus, the stapes, and the stapedius and tensor tympani muscles. The medial (labyrinthine) wall separates the middle and inner ears, and contains the oval and round windows, lateral semicircular canal, and tympanic segment of CN VII. The small hypotympanum is the inferior part of the tympanic cavity, below the cochlear promontory, and its floor separates the tympanic cavity from the jugular fossa.
The facial nerve (CN VII) enters the temporal bone via the porus acusticus/IAC. The labyrinthine segment extends from the fundus of the IAC to the geniculate ganglion (anterior genu). The nerve then turns posteriorly in its tympanic segment and, subsequently, turns vertically (posterior genu) to become the mastoid (descending) segment, exiting the skull base at the stylomastoid foramen.
The vestibule, semicircular canals, and cochlea form the bony labyrinth (otic capsule) of the inner ear. The vestibule is a large ovoid perilymphatic space, which connects anteriorly to the cochlea and to the three semicircular canals: superior, lateral (horizontal), and posterior. The cochlea is shaped like a cone, with its apex pointing anteriorly, laterally, and slightly down, consisting of 2.5 to 2.75 turns. The cochlea is optimally visualized in its entirety on the Stenver reconstructed image (subsequently described). The membranous labyrinth is, by definition, the fluid-filled space within the bony labyrinth that is filled by endolymph (including the endolymphatic duct and sac) and perilymph (within the cochlear duct).
CT of the temporal bone is usually performed with very thin sections (≤ 1.5 mm) and reformatted in Poschl (vestibular oblique view), Stenvers (cochlear oblique view), and standard axial and coronal planes, focusing on images reconstructed to display fine bony detail. Screening MR exams are performed with thin section (≤ 3 mm) technique, utilizing both the axial and coronal planes, with the strength of MR being depiction of soft tissue and fluid structures. The MR exam is typically supplemented with intravenous contrast injection, which is essential for evaluation of neoplastic disease, infection, and inflammation. For detail involving the temporal bone (and specifically the membranous labyrinth) as well as the adjacent CSF cisterns on MR, high resolution three-dimensional (3D) sequences, employing either constructive interference in the steady state (CISS) or fast spin-echo (FSE) techniques, are routinely acquired. At 3T images with a native isotropic resolution of ≤ 0.3 mm can be acquired in a clinically reasonable scan time.
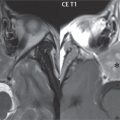
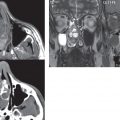
Congenital anomalies can involve the outer, middle, or inner ear. There can be stenosis or atresia of the external auditory canal. Dysplasia or aplasia can involve the middle or inner ear structures. Some malformations are known to be associated with meningitis, due to CSF leaks. The most common vascular variant is the dehiscent jugular bulb ( Fig. 2.6 ). In this variant, which typically presents with pulsatile tinnitus, there is a dehiscent sigmoid (jugular) plate and the jugular bulb extends to lie within the inferior tympanic cavity. It will appear to clinicians as a “vascular” TM.

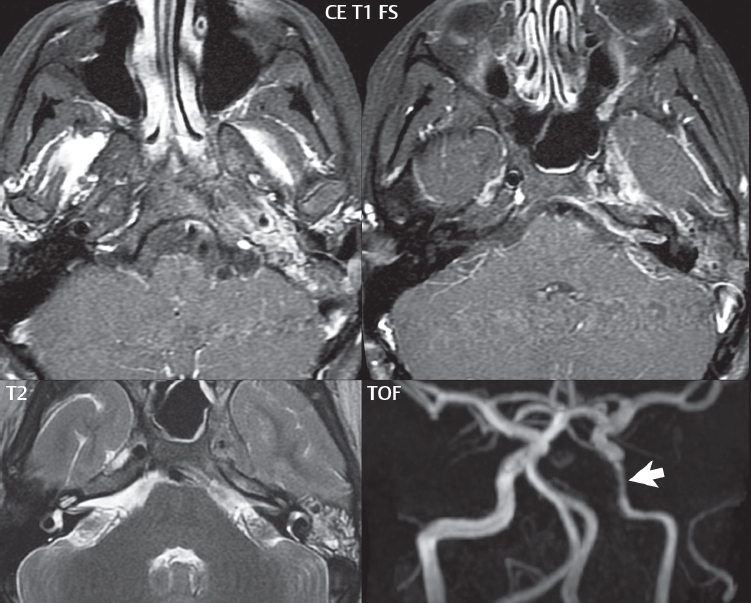
A large vestibular aqueduct, caused by enlargement of the endolymphatic sac and duct, is the most common anomaly associated with pediatric congenital sensorineural hearing loss ( Fig. 2.7 ). It is bilateral in 90% of patients and may be syndromic or nonsyndromic. The defining bony feature, as initially described, is enlargement of the vestibular aqueduct. There is a spectrum of associated cochlear and/or vestibular anomalies, ranging from subtle to gross dysmorphism. The most common specific features include modiolar deficiency and cochlear abnormalities. High-resolution T2-weighted MR (CISS or 3D FSE) is the imaging approach of choice, both for characterization and detection of associated anomalies. Dehiscence of the superior semicircular canal (SCC) (and much less commonly the posterior SCC) is being increasingly encountered. It is likely a developmental anomaly and patients typically present with Tullio phenomenon (sound-induced vertigo with or without nystagmus). Thin section, bone algorithm, high resolution temporal bone CT is used for evaluation, with Poschl reformatted images best to demonstrate this entity.

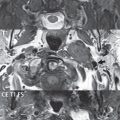
Inflammation and infection of the temporal bone can take on many different appearances. A middle ear effusion in an adult is important to recognize on both CT and MR, since eustachian tube obstruction, secondary to a nasopharynx neoplasm, must be excluded ( Fig. 2.8 ). Mastoiditis on CT has a nonspecific appearance, with opacification of mastoid air cells. On MR, the air cells are opacified with debris that is of intermediate, not high, signal intensity on T2-weighted scans, with prominent contrast enhancement ( Fig. 2.9 ). Complications associated with mastoiditis include sigmoid sinus thrombosis (with or without venous infarction), ( Fig. 2.10 ) Bezold abscess (an abscess in the sternocleidomastoid muscle), and intraparenchymal/epidural abscesses (middle and/or posterior cranial fossae) (Figs. 2.11 and 2.12). In chronic mastoiditis on CT, there will be demineralization of trabeculae, with eventual formation of one large cavity (coalescent mastoiditis). The specific subclassifications and procedures for mastoidectomy are complex and varied, with the term itself referring to resection of mastoid air cells ( Fig. 2.13 ). A mastoidectomy may be done to treat mastoiditis, chronic otitis media, or large cholesteatomas.





Labyrinthitis refers to inflammatory disease of the inner ear (specifically the membranous labyrinth), which can be secondary to a middle ear infection or meningitis. Of all infectious agents, a viral etiology is most common, resulting from upper respiratory infection. In this instance, the disease is usually self-limited and imaging is not performed. In acute and subacute labyrinthitis, CT is normal and the only imaging finding (which is still not seen in most patients) is faint enhancement of the fluid on MR within the labyrinth. Chronic labyrinthitis consists of a fibrous stage followed by an ossific stage. In the fibrous stage there is loss of the normal high signal intensity within the fluid-filled labyrinth on T2-weighted scans. In the ossific stage diffuse or focal ossification of the normally fluid-filled spaces is seen on CT, with hypointense signal therein on T2-weighted MR ( Fig. 2.14 ).

Patients with typical Bell palsy (herpetic peripheral CN VII paralysis) do not undergo imaging. Atypical cases are often evaluated with MR, not for imaging of the facial nerve, per se, but to exclude underlying pathology such as tumor. Paralysis of the facial nerve is thought to occur from latent herpes simplex infection of the geniculate ganglion, and is typically unilateral. On MR, the nerve in Bell palsy will be normal in diameter (occasionally slightly enlarged) and display uniform, linear enhancement, most common in the fundal and labyrinthine segments but, occasionally, throughout its entire course ( Fig. 2.15 ). Ramsey Hunt syndrome is caused by reactivation of a varicella zoster infection. As opposed to Bell palsy, however, it is typically associated with external ear vesicles, involvement of the entire intratemporal facial nerve and the vestibulocochlear nerve, with involvement of the membranous labyrinth ( Fig. 2.16 ). Patients present with CN VII palsy and sensorineural hearing loss.


Petrous apex lesions are varied and can be confusing. A cholesterol granuloma is the most common primary lesion of the petrous apex. This expansile lesion causes adjacent cortical thinning, and has distinctive high signal intensity on both T1- and T2-weighted images, the former due to the presence of hemorrhage and cholesterol. A cholesteatoma of the petrous apex (congenital or acquired) is less common but shares the characteristics of an expansile mass, with thinning and remodeling of adjacent bone. Its appearance on MR is, however, distinctive, consistent with its composition (an epidermoid), with low signal intensity on T1, high on T2, restricted diffusion, and no enhancement. Incidental lesions of the petrous apex that occasionally cause confusion include asymmetrical pneumatization and trapped fluid. The latter is common, and can be recognized by the presence of fluid with low T1 and high T2 signal intensity, without trabecular loss or any expansile nature ( Fig. 2.17 ). Apical petrositis has a distinct appearance, consistent with infection, with prominent enhancement, including the adjacent meninges ( Fig. 2.9 ). In the middle ear, the lesion of importance is the cholesteatoma. This lesion represents an enlarging mass of stratified squamous epithelium containing exfoliated keratin. The vast majority are acquired pars flaccida cholesteatomas. The appearance on CT is that of a soft tissue mass originating in Prussak space with erosion of the scutum and often filling the epitympanum ( Fig. 2.18 ). Bony destruction is characteristic with larger lesions with erosion of the ossicles and Koerner′s septum (a bony septum extending medially and inferiorly into the antrum) being common. On MR, a cholesteatoma has low T1 and high T2 signal intensity and manifests restricted diffusion. MR is commonly performed in postoperative cases for differentiation, on the basis of diffusion-weighted images, of residual cholesteatoma from other lesions.


The term “otosclerosis” is actually a misnomer as the condition is actually “otospongiosis.” There is resorption of the normal endochondral bony otic capsule (of unknown cause), with deposition of new spongy vascular bone. Clinically, these patients present with conductive hearing loss and bilateral disease in 80%. The majority of cases are fenestral in location, and can involve just the oval window or both the oval and round windows. Retrofenestral (cochlear) otospongiosis, which is less common, can be patchy or diffuse, and can occur with or without fenestral involvement. On CT, in the active phase of the disease, there is demineralization and, in the chronic phase, sclerosis. Treatment for the associated hearing loss includes hearing aids, early in the disease course, and, later, stapedectomy.
Cochlear implants are common today, with surgery performed for both congenital and acquired deafness, but require the presence of a functioning cochlear nerve. The electrode array is placed into the scala tympani (one of the two perilymph-filled cavities of the cochlear labyrinth) in the basal turn of the cochlea ( Fig. 2.19 ).


Although temporal bone fractures were traditionally categorized relative to their orientation to the long axis of the petrous bone, as longitudinal or transverse, the majority of fractures are actually complex or oblique. If the traditional categorization is used, the more common fracture is the longitudinal fracture which is parallel to the long axis of the petrous bone ( Fig. 2.20 ). Fractures can disrupt the ossicular chain, and transect either the cochlear nerve (with hearing loss) or facial nerve (with facial paralysis). CSF fistulas are not uncommon following a fracture involving the temporal bone.
Neoplasms
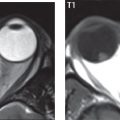
The most common neoplasm of the cerebellopontine angle is a vestibular schwannoma (arising from the vestibular division of CN VIII), which accounts for 10% of all intracranial tumors. Clinical symptoms consist of sensorineural hearing loss, tinnitus, and dysequilibrium. This benign, slow-growing, well delineated, encapsulated lesion typically arises within the internal auditory canal or (more commonly) is centered at the porus acusticus ( Fig. 2.21 ). Growth rates are variable. The typical presentation today, due to improved access to medical care and advanced imaging (MR), is that of a small lesion within the IAC. Presentation as a large mass lesion causing compression of the brainstem is uncommon ( Fig. 2.22 ). Although larger lesions can be seen on modern CT scanners, this modality does not play a clinical role in screening or diagnosis.


Vestibular schwannomas on MR are intensely enhancing lesions, identified by their location and focal nature. The majority have both an intra- and an extra-canalicular component, with mild enlargement of the porus acusticus and with the extra-canalicular portion rounded in appearance. Very large lesions can have a cystic component. Up to one-third of vestibular schwannomas are purely intracanalicular, and, thus, are very small lesions ( Fig. 2.23 ). For small to intermediate size lesions, stereotactic radiation is increasingly being recommended as the treatment of choice ( Fig. 2.24 ). Although much less common, schwannomas of the cochlear and facial nerves do occur ( Fig. 2.25 ). The latter can be, depending on extent, indistinguishable in appearance from a vestibular schwannoma. The differential diagnosis for a mass lesion at the cerebellopontine angle includes, in decreasing order of incidence, a (vestibular) schwannoma, meningioma, arachnoid cyst, and epidermoid.



Meningiomas can occur in the cerebellopontine angle; however, as opposed to vestibular schwannomas, they are usually located eccentric to the porus acusticus. A meningioma should not enlarge the porus acusticus, and will only, uncommonly, extend into the internal auditory canal ( Fig. 2.26 ). A broad-based lesion along a dural surface and calcification are common findings. Although a dural “tail” is a characteristic sign for a meningioma, this can also be seen with a vestibular schwannoma and is not a differentiating feature.

Epidermoids are very slow growing lesions, and can reach substantial size prior to clinical presentation (usually as an adult). An epidermoid tends to insinuate itself into the cisterns, incorporating cranial nerves and vessels, and causing mild (and sometimes massive) irregular compression of adjacent brain. On CT epidermoid tumors will manifest as “dirty” CSF. On MR these lesions are near isointensity with CSF, being only slightly hyperintense on FLAIR, but with distinctive hyperintensity on diffusion-weighted imaging (DWI). A little appreciated caveat is that the high signal intensity (SI) of an epidermoid on DWI is not due to restricted diffusion, but, rather, to T2 shine-through (thus the apparent diffusion coefficient [ADC] map will not show the lesion to have lower diffusion as compared to adjacent brain).
A trigeminal (CN V) schwannoma can arise anywhere along the course of the nerve, and, thus, can also occur at the cerebellopontine angle (CPA). A minority of trigeminal schwannomas in this location extend into both the middle and posterior fossae, having a dumbbell shape. The three major branches (ophthalmic, maxillary, and mandibular) of the trigeminal nerve exit the trigeminal ganglion (also known as the gasserian ganglion) which is located in the Meckel cave, with trigeminal schwannomas also rising in this location ( Fig. 2.27 ). Trigeminal schwannomas are much less common than vestibular schwannomas, with the latter accounting for 95% of intracranial schwannomas. Facial nerve and cochlear schwannomas ( Fig. 2.28 ) also occur, but are uncommon. The vast majority of CPA tumors are vestibular schwannomas (between 60 and 90%, in literature series), with other nonvestibular schwannomas much less common (4%).


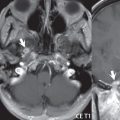
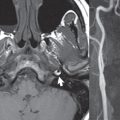
Head and neck paragangliomas occur in four common locations: in the tympanic cavity (glomus tympanicum paragangliomas [most common tumor of the middle ear]), at the skull base in the region of the jugular bulb (glomus jugulare paragangliomas [most common tumor of the jugular foramen]) ( Fig. 2.29 ), in the high retrostyloid parapharyngeal (carotid) space (glomus vagale paragangliomas), and at the common carotid artery bifurcation (carotid body paragangliomas). The latter tumor classically splays the ICA and ECA and completely “fills” the carotid bifurcation ( Fig. 2.30 ). Within the carotid sheath, glomus vagale paragangliomas displace the ICA anteriorly (as the vagus nerve is located posterior to the artery within the sheath) ( Fig. 2.31 ). However, some large glomus vagale paragangliomas extend caudally and can also splay the internal and external carotid arteries, much like a carotid body paraganglioma. The classic, more specific, location of a glomus tympanicum paraganglioma is on the cochlear promontory ( Fig. 2.32 ). This is the tumor known for its appearance as a red retrotympanic mass on otoscopic exam. One must be very careful to assess for presence of an aberrant internal carotid artery, which also presents with pulsatile tinnitus and a red retrotympanic mass. Look for integrity of the carotid canal wall. Large glomus tympanicum paragangliomas can fill the middle ear cavity ( Fig. 2.33 ).





Paragangliomas, other than the glomus tympanicum paragangliomas, are typically smoothly contoured and ovoid in shape. Large tumors may be inhomogeneous, with areas of necrosis. On both T1- and T2-weighted images, the classic appearance is that of “salt and pepper,” with tiny areas of high signal intensity (salt) due to slow flow (or hemorrhage), and low signal intensity (pepper) due to fast flow (flow voids). Paragangliomas are hypervascular tumors, with early prominent enhancement. Digital subtraction angiography (DSA) reveals enlarged feeding arteries, and rapid venous drainage. Contrast enhancement on MR, in addition to providing lesion characterization (for all paragangliomas), assists in detection of small glomus tympanicum paragangliomas. However, this is typically not required as a noncontrast temporal bone CT is usually diagnostic (soft tissue mass on the cochlear promontory) and best to exclude a potential aberrant ICA. Bone destruction is common with glomus jugulare paragangliomas and is classically permeative-destructive. Glomus jugulare paragangliomas may also extend into the internal jugular vein. Although much less common, a schwannoma or meningioma can occur in the region of the jugular foramen, and should be kept in mind in terms of differential diagnosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree