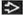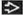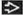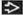(T) Primary Tumor | Adapted from 7th edition AJCC Staging Forms. | |
TNM | Definitions | |
pTX | Primary tumor cannot be assessed (if no radical orchiectomy has been performed, TX is used) | |
pT0 | No evidence of primary tumor (e.g., histologic scar in testis) | |
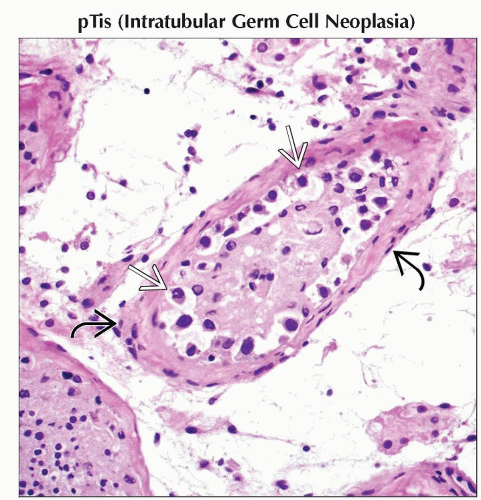
pTis | Intratubular germ cell neoplasia (carcinoma in situ) | |
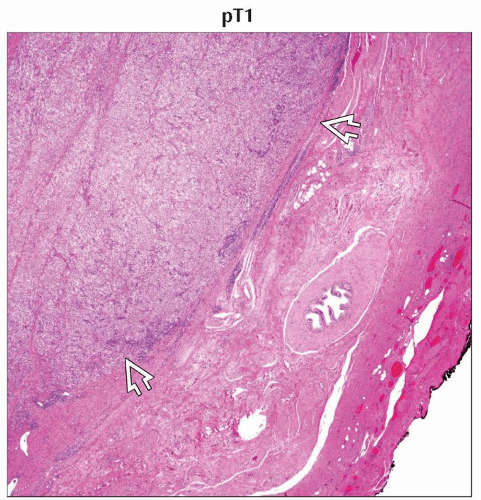
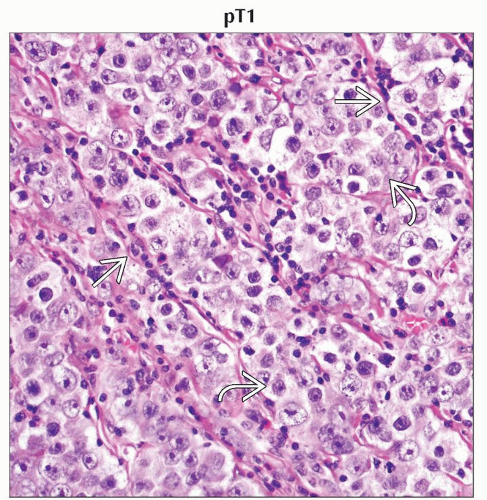
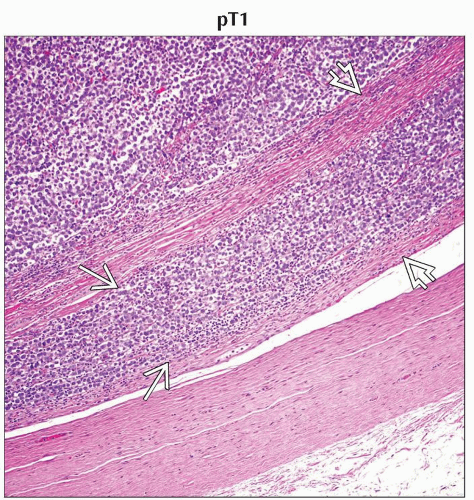
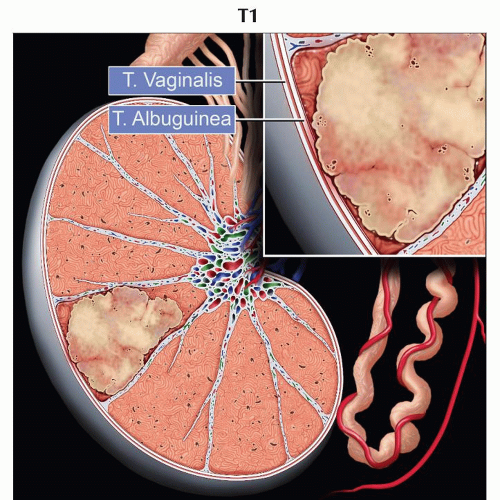
pT1 | Tumor limited to the testis and epididymis without vascular/lymphatic invasion; tumor may invade into the tunica albuginea but not the tunica vaginalis | |
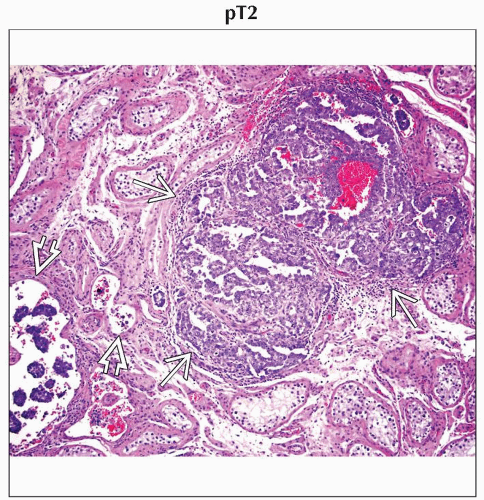
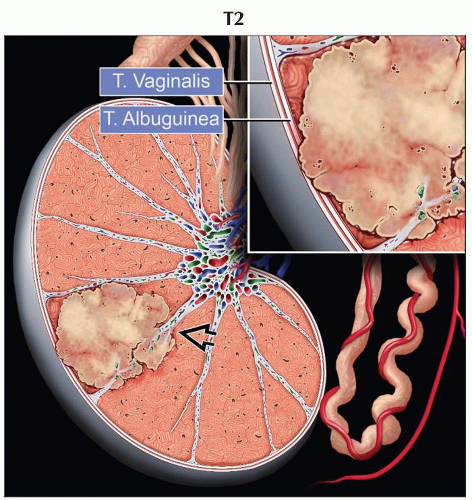
pT2 | Tumor limited to the testis and epididymis with vascular/lymphatic invasion, or tumor extending through the tunica albuginea with involvement of the tunica vaginalis | |
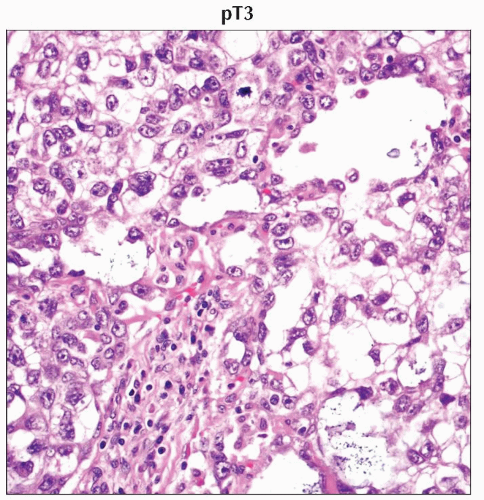
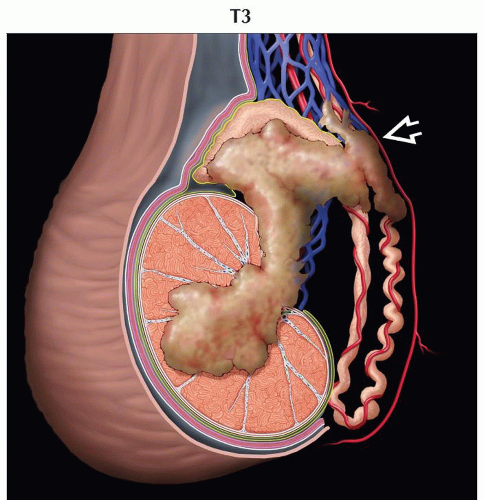
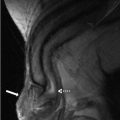
pT3 | Tumor invades the spermatic cord with or without vascular/lymphatic invasion | |
pT4 | Tumor invades scrotum with or without vascular/lymphatic invasion | |
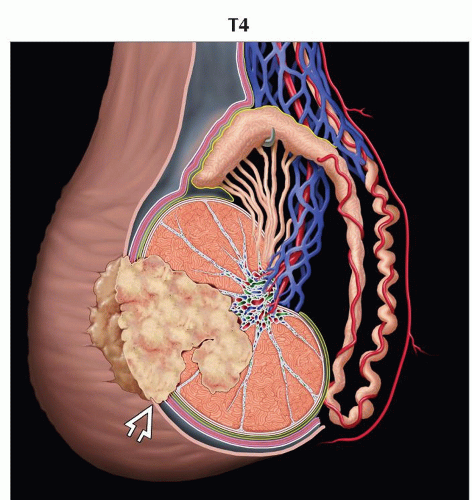
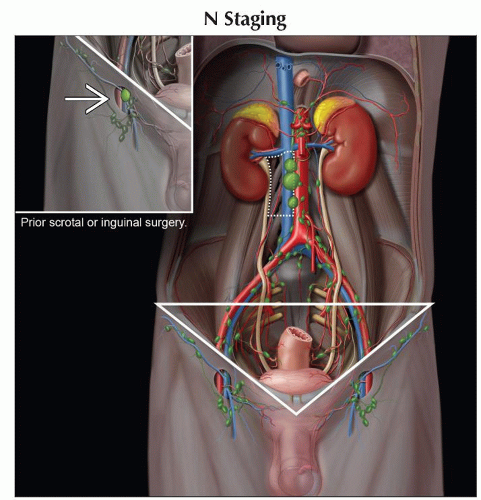
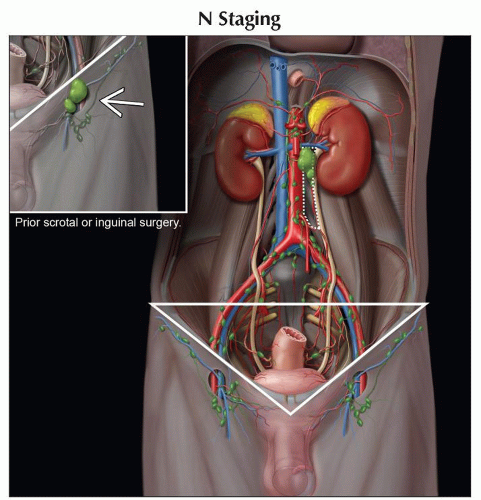
(N) Regional Lymph Nodes | ||
Clinical | ||
NX | Regional lymph nodes cannot be assessed | |
N0 | No regional lymph node metastasis | |
N1 | Metastasis with a lymph node mass ≤ 2 cm in greatest dimension; or multiple lymph nodes, none > 2 cm in greatest dimension | |
N2 | Metastasis with a lymph node mass > 2 cm but ≤ 5 cm in greatest dimension; or multiple lymph nodes, any 1 mass > 2 cm but ≤ 5 cm in greatest dimension. | |
N3 | Metastasis with a lymph node mass > 5 cm in greatest dimension | |
Pathological | ||
pNX | Regional lymph nodes cannot be assessed | |
pN0 | No regional lymph node metastasis | |
pN1 | Metastasis with a lymph node mass ≤ 2 cm in greatest dimension and ≤ 5 nodes positive, none > 2 cm in greatest dimension | |
pN2 | Metastasis with a lymph node mass > 2 cm but ≤ 5 cm in greatest dimension; or > 5 nodes positive, none > 5 cm; or evidence of extranodal extension of tumor | |
pN3 | Metastasis with a lymph node mass > 5 cm in greatest dimension | |
(M) Distant Metastasis | ||
M0 | No distant metastasis | |
M1 | Distant metastasis | |
M1a | No regional nodal or pulmonary metastasis | |
M1b | Distant metastasis other than to nonregional lymph nodes and lungs | |
Except for pTis and pT4, extent of primary tumor is classified by radical orchiectomy. For this reason, a pathologic stage is usually assigned. TX may be used for other categories in the absence of radical orchiectomy. | ||
Serum Tumor Markers (S) | |
TNM | Definitions |
SX | Tumor marker studies not available or not performed |
S0 | Tumor marker study levels within normal limits |
S1 | LDH < 1.5x normal and β-hCG < 5,000 IU/L and AFP < 1,000 ng/mL |
S2 | LDH 1.5-10x normal or β-hCG 5,000-50,000 IU/L or AFP 1,000-10,000 ng/mL |
S3 | LDH >10x normal or β-hCG > 50,000 IU/L or AFP > 10,000 ng/mL |
Serum tumor markers are used as part of tumor stage grouping in testicular cancer. LDH = lactate dehydrogenase, hCG = human chorionic gonadotropin, AFP = α-fetoprotein. | |
AJCC Stages/Prognostic Groups | Adapted from 7th edition AJCC Staging Forms. | ||||
Stage | T | N | M | S (Serum Tumor Markers) | |
0 | pTis | N0 | M0 | S0 | |
I | pT1-4 | N0 | M0 | SX | |
IA | pT1 | N0 | M0 | S0 | |
IB | pT2 | N0 | M0 | S0 | |
pT3 | N0 | M0 | S0 | ||
pT4 | N0 | M0 | S0 | ||
IS | Any pT/TX | N0 | M0 | S1-3 (measured post orchiectomy) | |
II | Any pT/TX | N1-3 | M0 | SX | |
IIA | Any pT/TX | N1 | M0 | S0 | |
Any pT/TX | N1 | M0 | S1 | ||
IIB | Any pT/TX | N2 | M0 | S0 | |
Any pT/TX | N2 | M0 | S1 | ||
IIC | Any pT/TX | N3 | M0 | S0 | |
Any pT/TX | N3 | M0 | S1 | ||
III | Any pT/TX | Any N | M1 | SX | |
IIIA | Any pT/TX | Any N | M1a | S0 | |
Any pT/TX | Any N | M1a | S1 | ||
IIIB | Any pT/TX | N1-3 | M0 | S2 | |
Any pT/TX | Any N | M1a | S2 | ||
IIIC | Any pT/TX | N1-3 | M0 | S3 | |
Any pT/TX | Any N | M1a | S3 | ||
Any pT/TX | Any N | M1b | Any S | ||
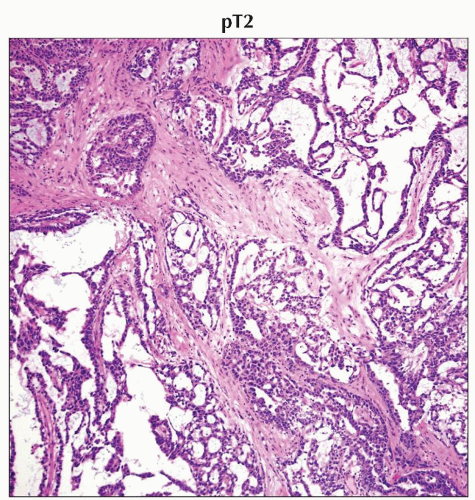 In an H&E-stained specimen from the same patient, other areas of the mixed germ cell tumor show yolk sac component. (Original magnification 400x.) |
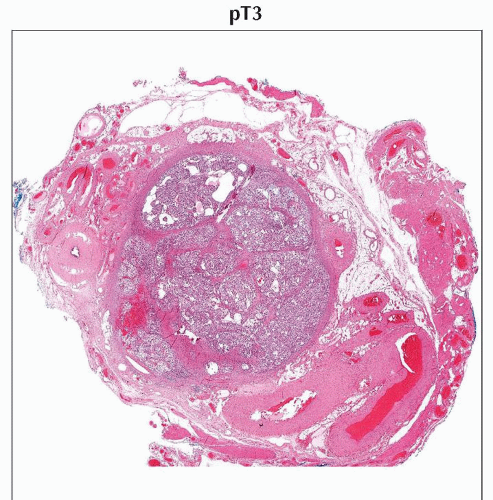 H&E stain of a cross section from a spermatic cord shows spermatic cord involvement by a testicular tumor. (Original magnification 20x.) |
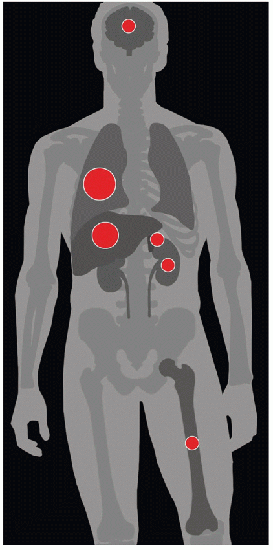
| METASTASES, ORGAN FREQUENCY | |
Lungs | 89% | |
Liver | 73% | |
Brain | 31% | |
Bone | 30% | |
Kidneys | 30% | |
Adrenal | 29% | |
Data from Bredael JJ et al: Autopsy findings in 154 patients with germ cell tumors of the testis. Cancer. 50(3):548-51, 1982. | ||
Germ cell tumors constitute 95% of all testicular tumors
Intratubular germ cell neoplasia, unclassified
Malignant pure germ cell tumor (showing single cell type)
Seminoma
Embryonal carcinoma
Teratoma
Choriocarcinoma
Yolk sac tumor
Malignant mixed germ cell tumor (showing > 1 histologic pattern)
Embryonal carcinoma and teratoma ± seminoma (teratocarcinoma)
Embryonal carcinoma and yolk sac tumor ± seminoma
Embryonal carcinoma and seminoma
Yolk sac tumor and teratoma ± seminoma
Choriocarcinoma and any other element
Polyembryoma
Sex cord and stromal tumors
Leydig cell tumor
Sertoli cell tumor
Granulosa cell tumor
Fibroma-thecoma
Tumors with both sex cord and stromal cells and germ cells
Gonadoblastoma
Direct extension
Through tunica albuginea with involvement of scrotal skin is rare and late finding
Lymphatic spread
Most germ cell tumors spread 1st via lymphatics rather than hematogenously
Choriocarcinoma is notorious for early hematogenous spread
Lymphatic involvement occurs in predictable stepwise fashion
Right testis → right-sided nodes around inferior vena cava (IVC) (most commonly lower retroperitoneal, aortocaval, or paracaval)
Left testis → left paraaortic nodes in area bounded by renal vein, aorta, ureter, and inferior mesenteric artery
In absence of bulky ipsilateral adenopathy, contralateral spread is unusual
If seen as only site of metastasis, histological proof of tumor involvement should be sought prior to instituting therapy
Pelvic adenopathy is uncommon in absence of bulky disease elsewhere
Inguinal lymph node metastases from testicular tumors are reported in 2% of cases, primarily to ipsilateral inguinal nodes
Pattern is seen in 2 groups of patients
Lymphatic disruption from prior scrotal or inguinal surgery
Tumor-contaminated scrotum
If patient did not have scrotal or inguinal surgery, nor scrotal invasion or contamination
Inguinal nodes are then considered nonregional (M1 disease)
Nodal disease superior to level of renal hila occurs via direct spread
With seminoma, spread of disease above diaphragm may occur via thoracic duct into posterior mediastinum
With nonseminomatous germ cell tumor (NSGCT), spread is more random involving anterior mediastinum, aortopulmonary window, hilar, supraclavicular, and neck lymph nodes
Excludes posterior mediastinum and subcarinal regions
Hematogenous spread
Occurs late, except in case of choriocarcinoma
Lung is most common location (89%); other sites include liver (73%), brain (31%), bone (30%), kidney (30%), and adrenal (29%)
Comments
< 50% of malignant testicular germ cell tumors have single cell type
˜ 50% are seminomas
Remaining tumors have > 1 cell type, and relative proportions of each cell type should be specified
Cell type of these tumors is important for estimating risk of metastases and response to chemotherapy
“Burned-out” tumor describes regressed occult gonadal germ cell tumor with primary metastases
Primary testicular tumor is reduced to fibrotic scar, sometimes with intratubular cancerous germ cells
May account for 10% of apparently primary retroperitoneal germ cell tumors
Etiology
Well-established associations include
Prior testicular tumor
Increases risk of contralateral tumor by 20x
Positive family history
1st-degree relative with testicular carcinoma increases risk by 6x
Cryptorchidism
Present in 3.5-14.5% of patients with testicular carcinoma
Infertility
Testis biopsies in subfertile men show 0.4-1.1% prevalence of intratubular germ cell neoplasia, excluding cases of cryptorchidism
Microlithiasis remains somewhat controversial as a risk factor
Ultrasound definition of at least 5 microliths on 1 image
Increased relative risk of testicular carcinoma in setting of microlithiasis is 2-20x
In setting of microlithiasis without mass, majority of current recommendations are for monthly self exams without routine ultrasound screening
Small increased relative risk of testicular carcinoma has been reported in patients with history of mumps orchitis
Epidemiology & cancer incidence
Increasing prevalence, > 100% increase in reported cases since 1938
Annual percentage change +2.3% between 1975 and 1989, +0.8% between 1989 and 2005
Most common neoplasm in males 15-44 years old
Median age at diagnosis: 34 years old
4.5x more common in Caucasians than African-Americans
Age-adjusted incidence of 5.4/100,000
Estimated 8,400 new cases in USA in 2009
Age-adjusted death rate of 0.3/100,000
Estimated 380 deaths in USA in 2009
1 of most curable tumors
5-year survival rate from 1996-2004 statistics was 95.5%
H&E
Seminoma
Uniform cellular morphology resembling primitive germ cells
Embryonal cell carcinoma
Papillary tumor with primitive anaplastic epithelial cells resembling early embryonic cells
Teratoma
Variable appearance with possible ciliated epithelial-lined cysts, densely staining bone and cartilage, and mucin-producing glandular structures
Choriocarcinoma
Pale staining cytotrophoblasts surrounded by syncytiotrophoblasts
“Burned-out” germ cell tumor
Scarring with homogeneous sparsely cellular collagen
Special stains
Yolk sac tumor
Anti-α-fetoprotein stain positive with brownish deposits within tumor cells
Ultrasound
Primary imaging modality for evaluation of testicular lesions
Nearly 100% sensitivity for identification of scrotal masses; can almost always differentiate intratesticular from extratesticular mass
Ultrasound cannot reliably differentiate tumor subtypes, and orchiectomy is required for detailed pathologic evaluation of tumor
Most testicular tumors are hypoechoic to surrounding normal parenchyma
Some tumors can be heterogeneous with cystic areas and calcifications
Larger tumors tend to be more vascular
Ultrasound appearance of different histologic types
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree