LEARNING OBJECTIVES
1. Imaging findings and evolution of biopsy-related changes
2. Imaging findings of trauma-related changes in the breast
3. Imaging findings and evolution of changes expected following lumpectomy and radiation therapy
4. Medical complications related to breast hypertrophy
5. Imaging findings associated to reduction mammoplasty
6. Imaging women with implants
7. Imaging implant-related complications
EXCISIONAL BIOPSY CHANGES
Accurate pre-operative wire localization methods facilitate minimal volume biopsies and an increasing number of minimally invasive imaging-guided needle biopsies have reduced the need for excisional biopsies. Consequently, the likelihood of an excisional biopsy creating a permanent change that affects subsequent mammographic interpretations has decreased significantly. When post-operative changes occur, they often resolve in the first several months after the procedure. Unless a mammogram is done in the first 6 months following a biopsy, annual mammograms are normal in over 50% of these women (1–3) and, in those women in whom a change is seen, it is often recognizable as related to a biopsy particularly when correlated with history to the site of the biopsy.
The technologists take a careful history, inspect the breast, and document the site of any scars on the patient’s history sheet. This allows the interpreting radiologist to correlate any findings on the mammogram with prior biopsy sites. Although we do not routinely mark biopsy scars at the time screening studies are done, thin wires or metallic BBs can be used (2). Given the expense associated with these markers, in conjunction with the high number of women who have had at least one breast biopsy, we reserve the use of markers for the few patients a year in whom there is a question about an area of mammographic concern correlating with a biopsy site. Most importantly, when reviewing images, we want to minimize distractions that may interfere with our ability to identify early-stage breast cancers; metallic makers (e.g., for nipples) and wires are a distraction that, in most patients, provide no useful diagnostic information (Fig. 11.1).
Changes that may be seen on a mammogram correlating with excisional biopsy sites include skin thickening and retraction, distortion (Fig. 11.2) that is often seen best in one of the standard views and subtle or not apparent in the other view, and a round, oval, or irregular mass possibly fat-containing with spiculated or indistinct margins (Fig. 11.3) (4). Depending on the amount of tissue excised, generalized observations may include asymmetry in the size of the breasts with the operated breast being smaller and parenchymal asymmetry such that less tissue is present at the surgery site compared with remaining tissue at the corresponding site in the contralateral breast (Fig. 11.4). Except for the asymmetric breast size and resulting parenchymal asymmetry seen in some patients, focal changes usually resolve completely on serial mammograms, remain stable after the first year or slowly evolve with time (Fig. 11.5). Increasing amounts of fat centrally in a mass, dispersion of the density, oil cyst formation, and the development of dystrophic calcifications are changes that can be seen on follow up (1–4). Occasionally, retained fragments of the localization wire (Fig. 11.6A), needle tips, or foreign bodies (Fig. 11.6B) may be seen at prior surgical sites.
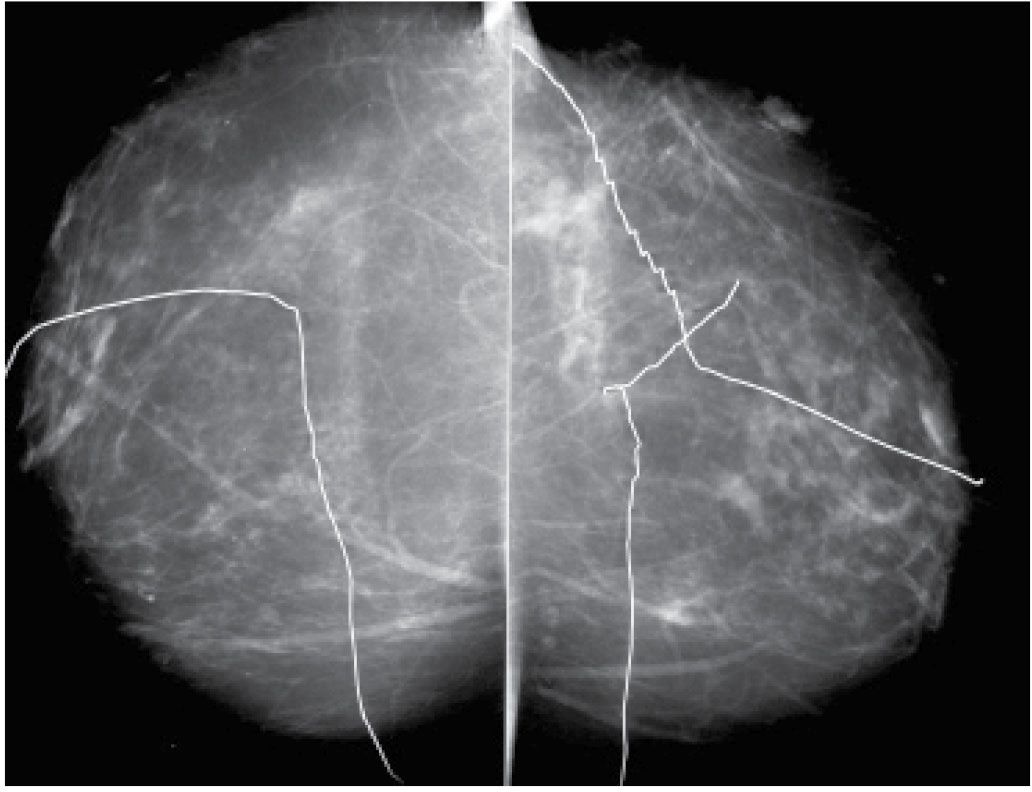
FIG. 11.1 • Biopsy markers. CC views. Wires used to mark prior incisions for a reduction mammoplasty. The first things you notice are the wires distracting you from the task at hand. Given the potential for distraction, lack of changes related to prior biopsies in more than 50% of patients, and the cost of the markers, we do not routinely mark prior biopsy sites. Even in patients with post-biopsy changes, the findings are distinctive enough that, in combination with the history, a confident diagnosis is made without the use of markers. For similar reasons, we do not use nipple markers. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)
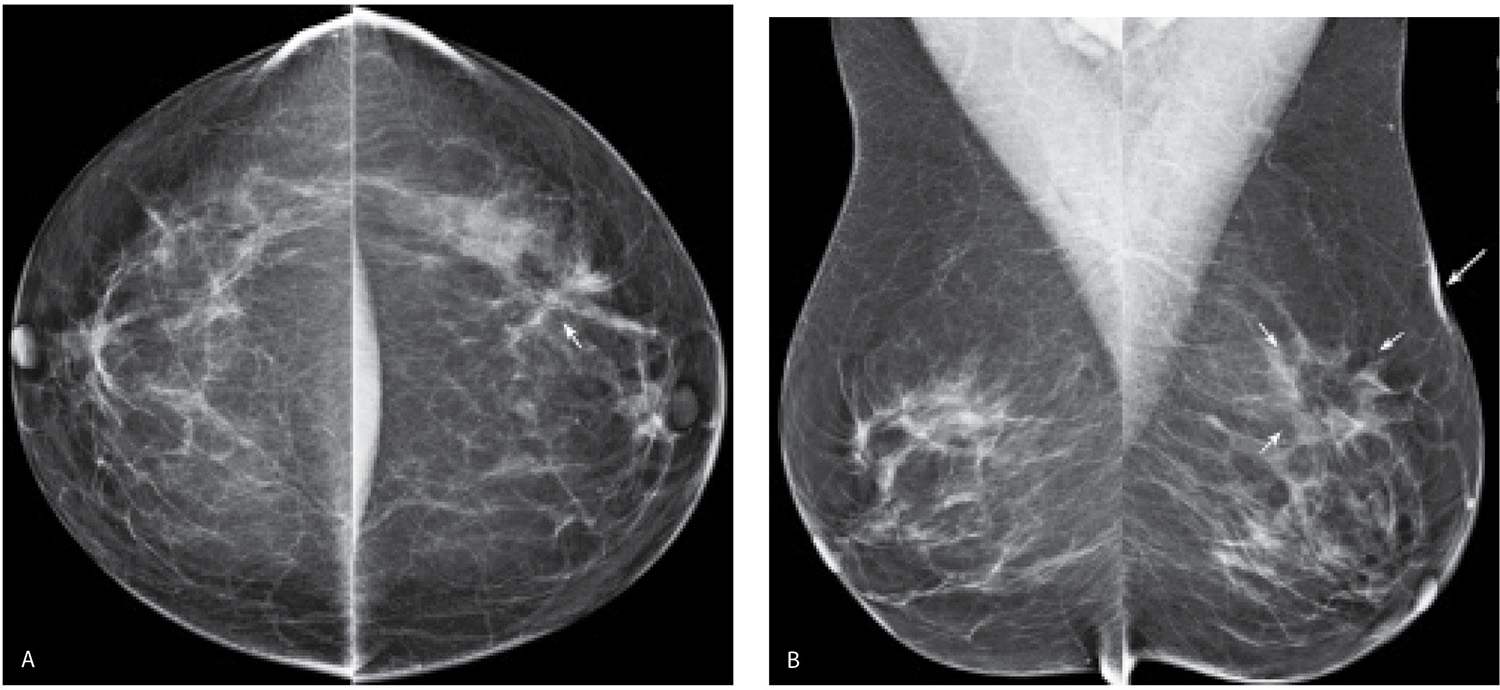
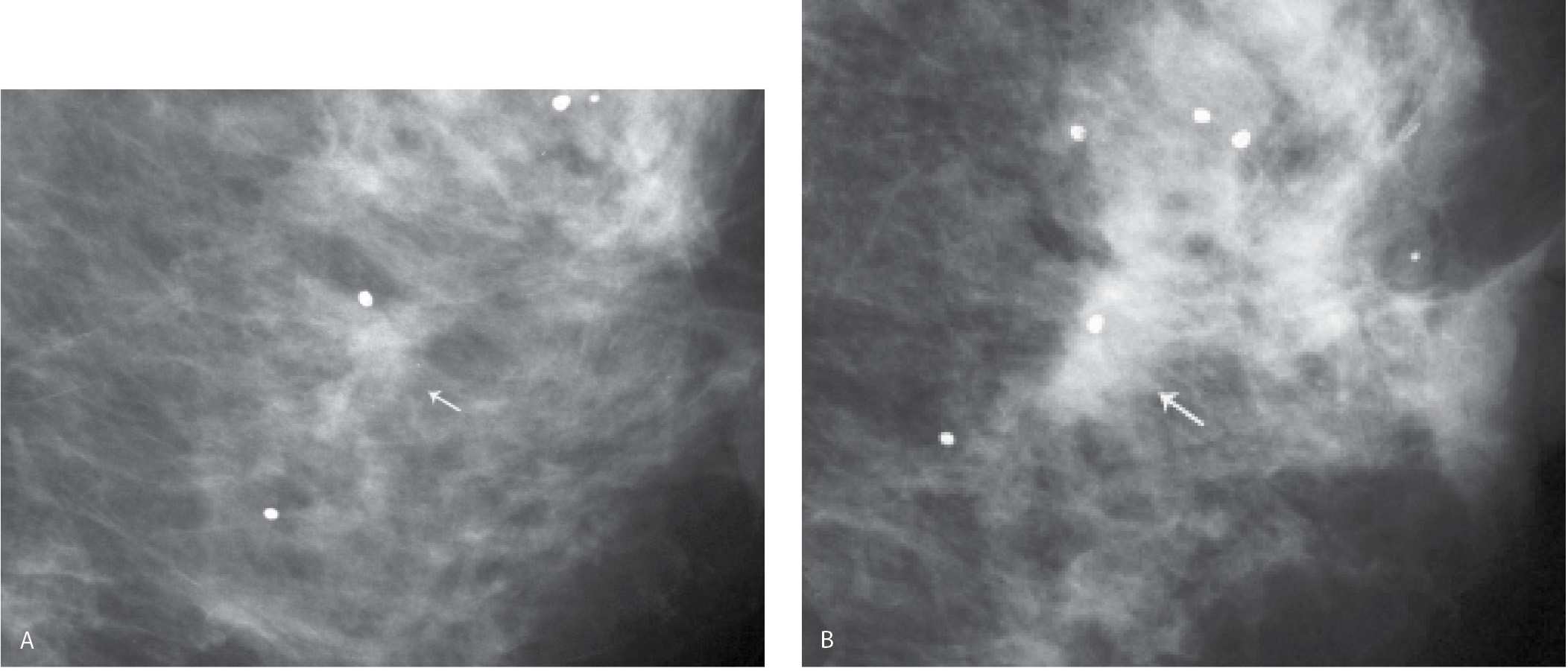
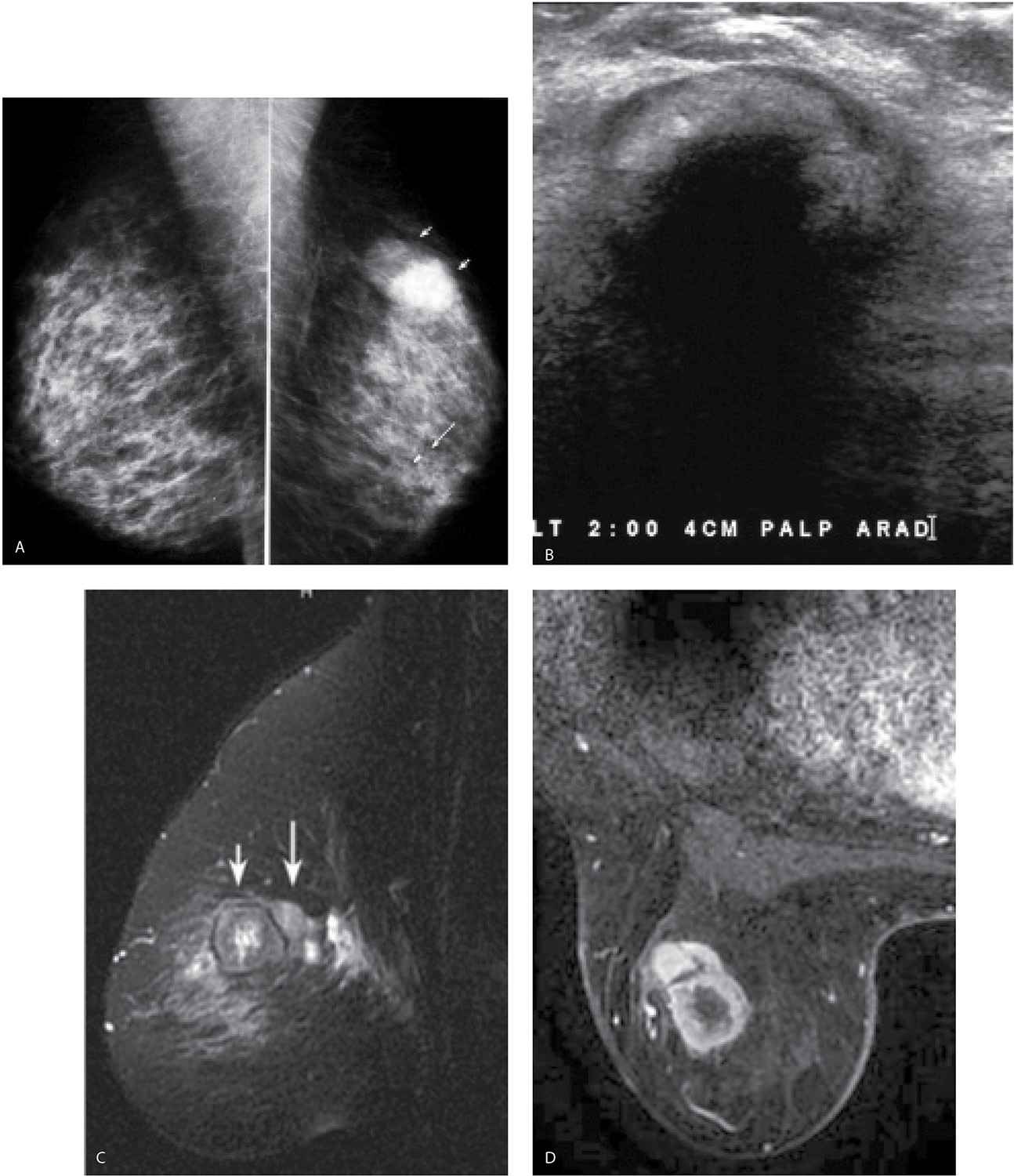
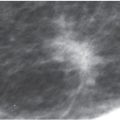
FIG. 11.2 • Biopsy-related changes, left breast. CC (A) and MLO (B) views in a 48-year-old woman 3 years after an excisional biopsy of the left breast. Distortion (arrow) is apparent laterally in the left breast on the CC projection. Focal skin thickening (long arrow) and distortion (short arrows) on the MLO view, the overall configuration and fat content of which, is different from the appearance of this area on the CC view. When distortion is seen more prominently in one of the two standard projections, review the patient’s history for prior breast surgery. Reviewing the mammograms of women who have had prior excisional biopsies is a good way to teach yourself how to detect subtle areas of distortion.
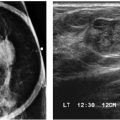
When biopsy changes are noted mammographically and correlated to a biopsy site, evaluation with ultrasound or magnetic resonance imaging (MRI) is not usually indicated. Ultrasound and MRI findings, however, will vary significantly depending on the timing of these studies to the biopsy. Acutely, biopsy changes on ultrasound may be characterized by increases in the echogenicity of the tissue, loss of normal soft tissue planes, fluid collections (Fig. 11.7A) that may be complex and skin thickening. Distortion and shadowing (5) that may be seen best in one plane and become less apparent as the transducer is rotated 90-degrees are common findings at long-standing biopsy sites.
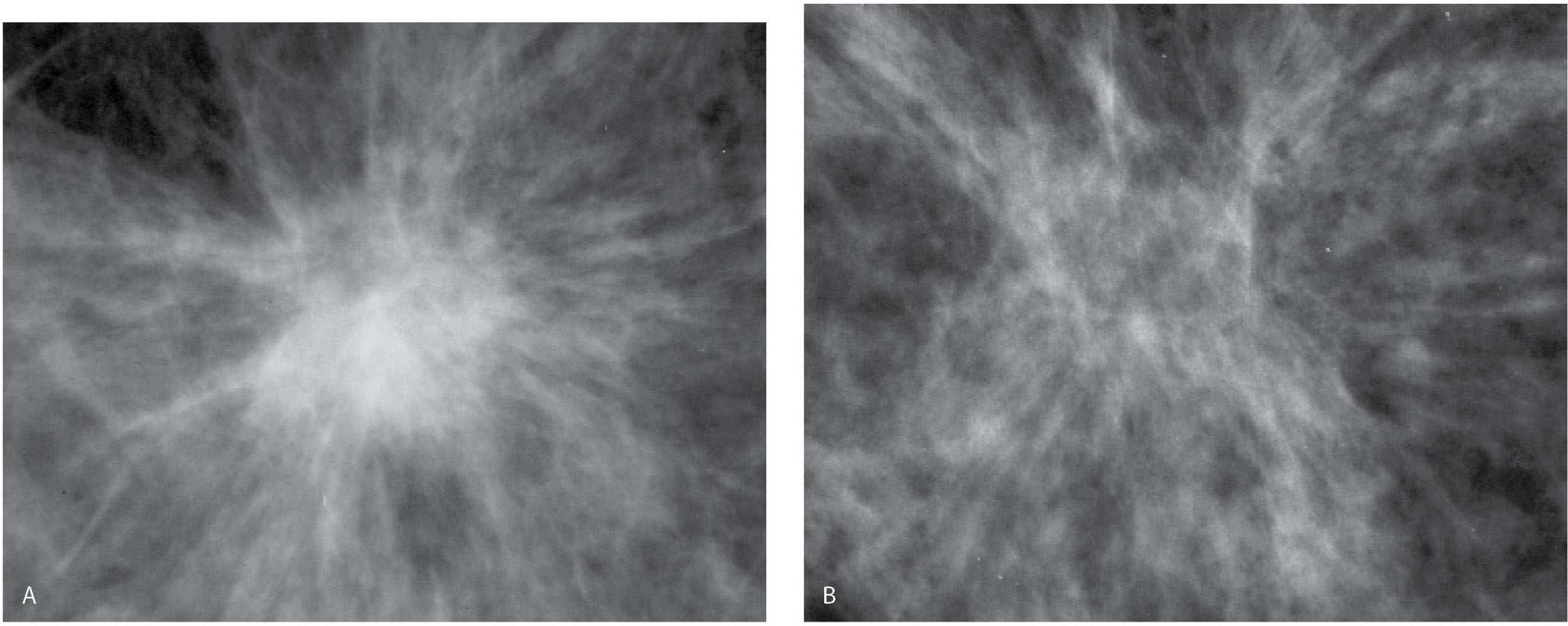
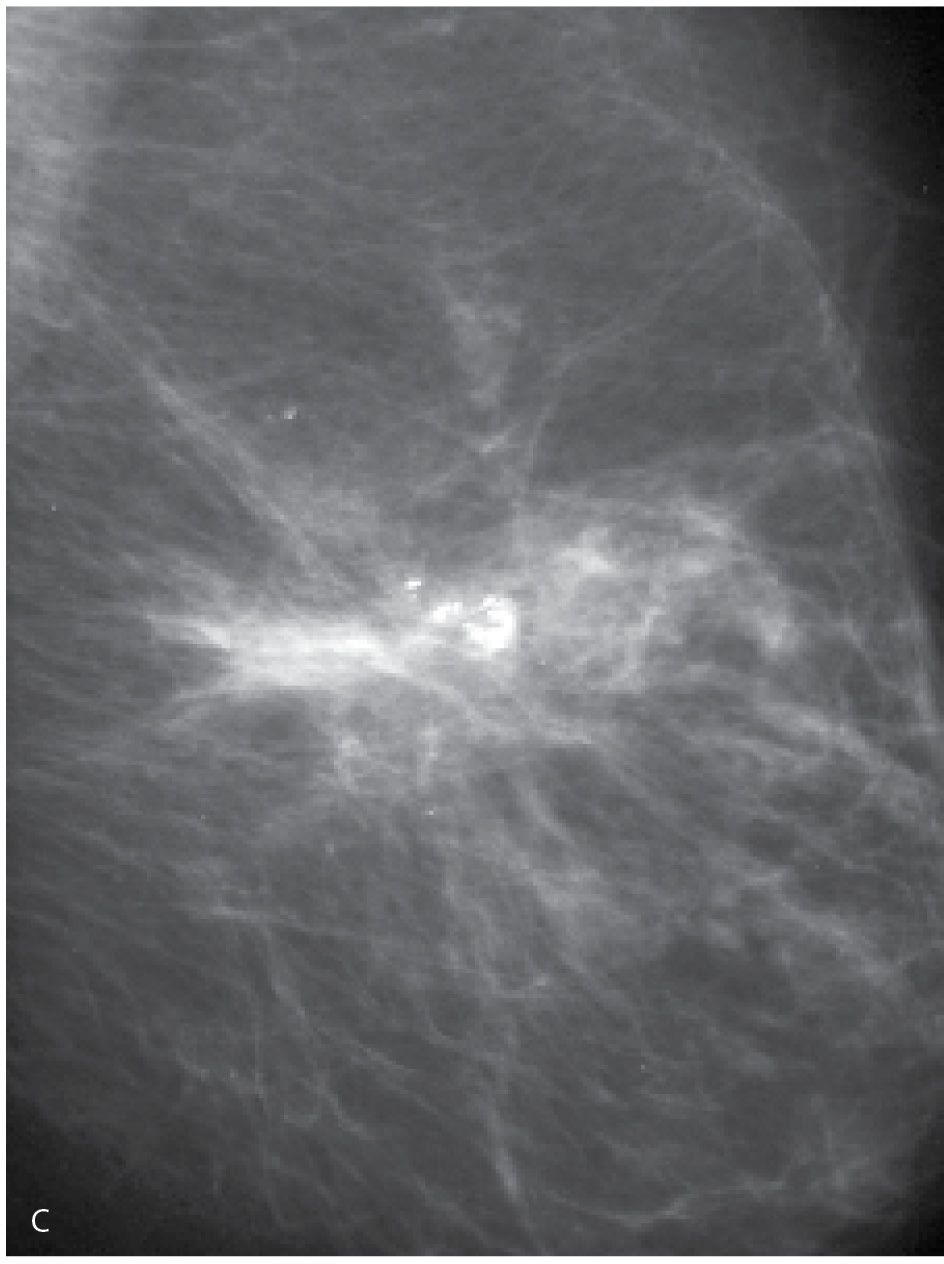
FIG. 11.3 • Biopsy-related fat necrosis. A: An isodense round mass with spiculated margins is imaged corresponding to a prior biopsy site. B: On a follow-up study, the previously noted central density (mass) has been replaced with fat and the remaining finding is best described as distortion. This may stabilize, or if the soft tissue component continues to resolve, an oil cyst and dystrophic calcifications may develop. You should use biopsy changes such as these to test your ability to detect subtle areas of distortion and spiculation. If you can detect these findings in patients who have had a biopsy, you will probably detect small, subtle breast cancers presenting with distortion on screening studies. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)
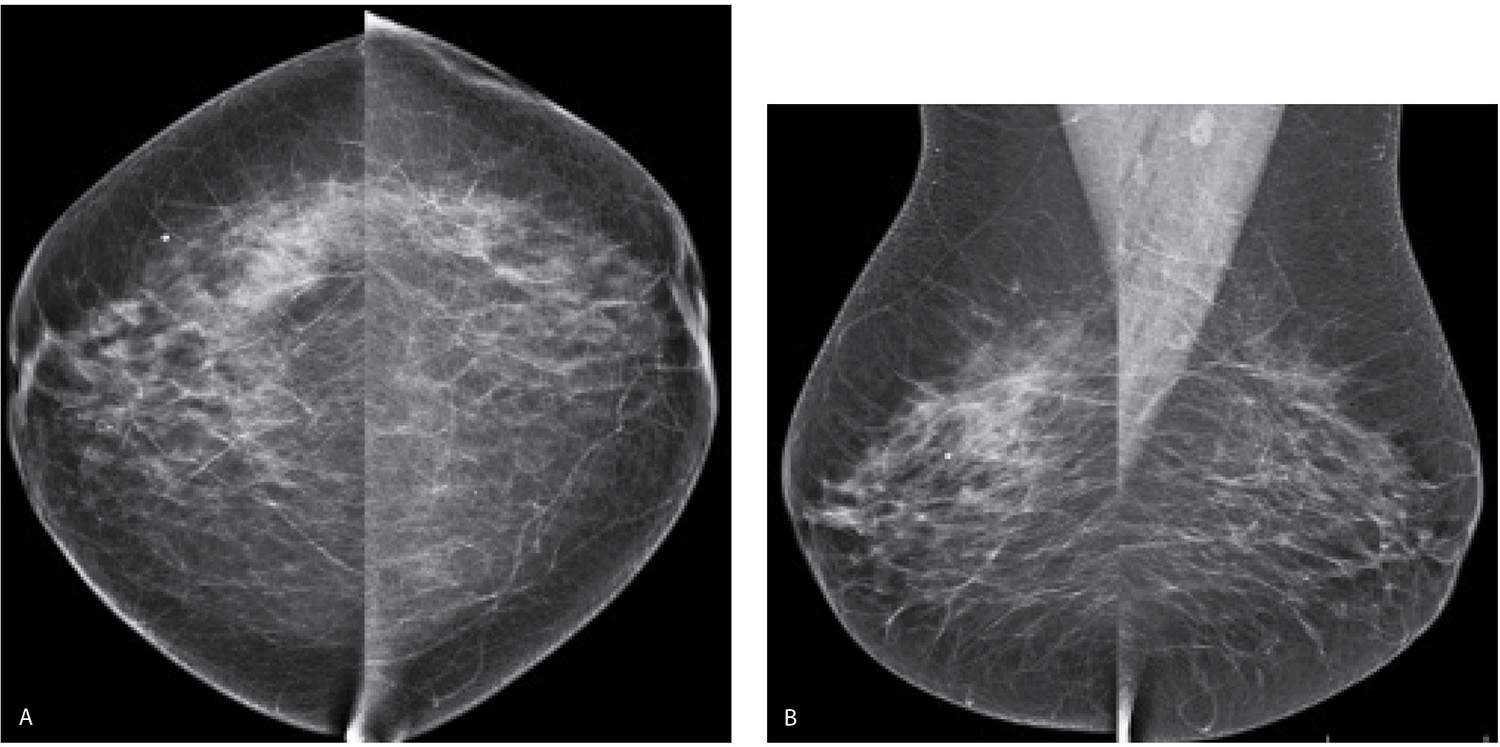
FIG. 11.4 • Biopsy changes, left breast. CC (A) and MLO (B) views in a 68-year-old woman many years following an excisional biopsy of the left breast. Parenchymal asymmetry is evident with less tissue noted in the upper outer quadrant of the left breast. Subtle skin changes are noted laterally in the left CC and distortion is noted superiorly in the left oblique view.
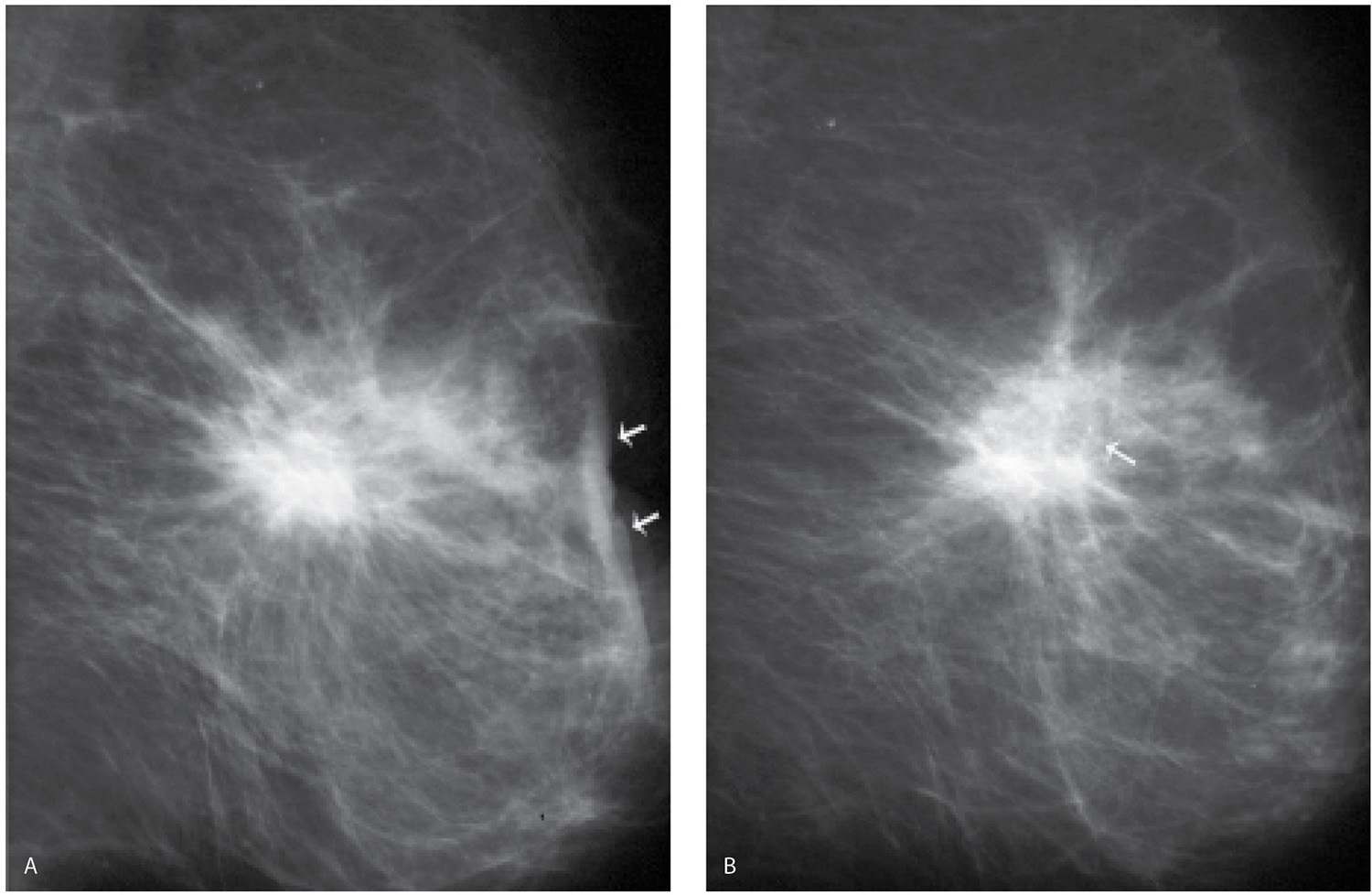
FIG. 11.5 • Evolution of biopsy changes. A: MLO view photographically coned to the anterior aspect of the left breast obtained 2 months following an excisional biopsy. A mass with spiculated margins and associated distortion is noted correlating to the excisional biopsy site. Skin changes including thickening, distortion, and retraction are present (arrows). B: Six months following the image in part A, 8 months following the biopsy. The mass persists, however, is smaller and less dense. A lucent area is developing in the center (arrow) associated with some punctate calcifications. Skin changes are less prominent. C: One year following the biopsy. The overall size and shape of the remaining density is changed compared with the mass seen in A and B. Distortion persists and dense, coarse calcifications are now present centrally. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)
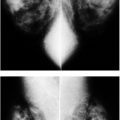
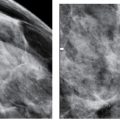
On MRI, an irregular mass with mural or internal areas of high T1 and T2 signal and minimal or no enhancement is a common finding for acute hematomas (Fig. 11.7B–D; also see Fig. 5.9); if enhancement is present, it is often thin and peripheral in location. Subacute and chronic post-operative fluid collections may demonstrate a heterogeneously high T2 signal with intermediate to low T1 signal and internal non-enhancing nodules. Minimal enhancement may be noted involving the wall of the collection. Following re-absorption of post-operative fluid collections, increased non-enhancing soft tissue with distortion, an associated fat signal and skin changes (retraction, focal thickening) may be apparent.
Post-operative changes are characterized by progressive resolution or stability needs to be established on annual studies. This is particularly important in patients with a high-risk lesion (e.g., atypical ductal hyperplasia, lobular neoplasia, and multiple peripheral papillomas) diagnosed on excisional biopsy, or those with a personal or significant family history of breast cancer. In these patients, a mammogram 6 months after the biopsy is helpful in establishing the presence of any biopsy change (e.g., a new baseline for the patient). Changes seen 6 months after a biopsy are unlikely to represent recurrent or interval cancer. On subsequent studies, however, we expect the changes to stabilize or, more commonly, evolve, becoming less prominent with time. Increases in distortion or density at a biopsy site after the initial 6-month post-operative study warrant a biopsy recommendation and a review of the original pathology (Fig. 11.8). As yearly studies accrue on a patient, comparison is made to the earliest post-operative study available. Subtle progressive changes may not be readily apparent from year to year, but can be quite obvious, if comparison is made to the earliest available post-operative study (Fig. 11.9).
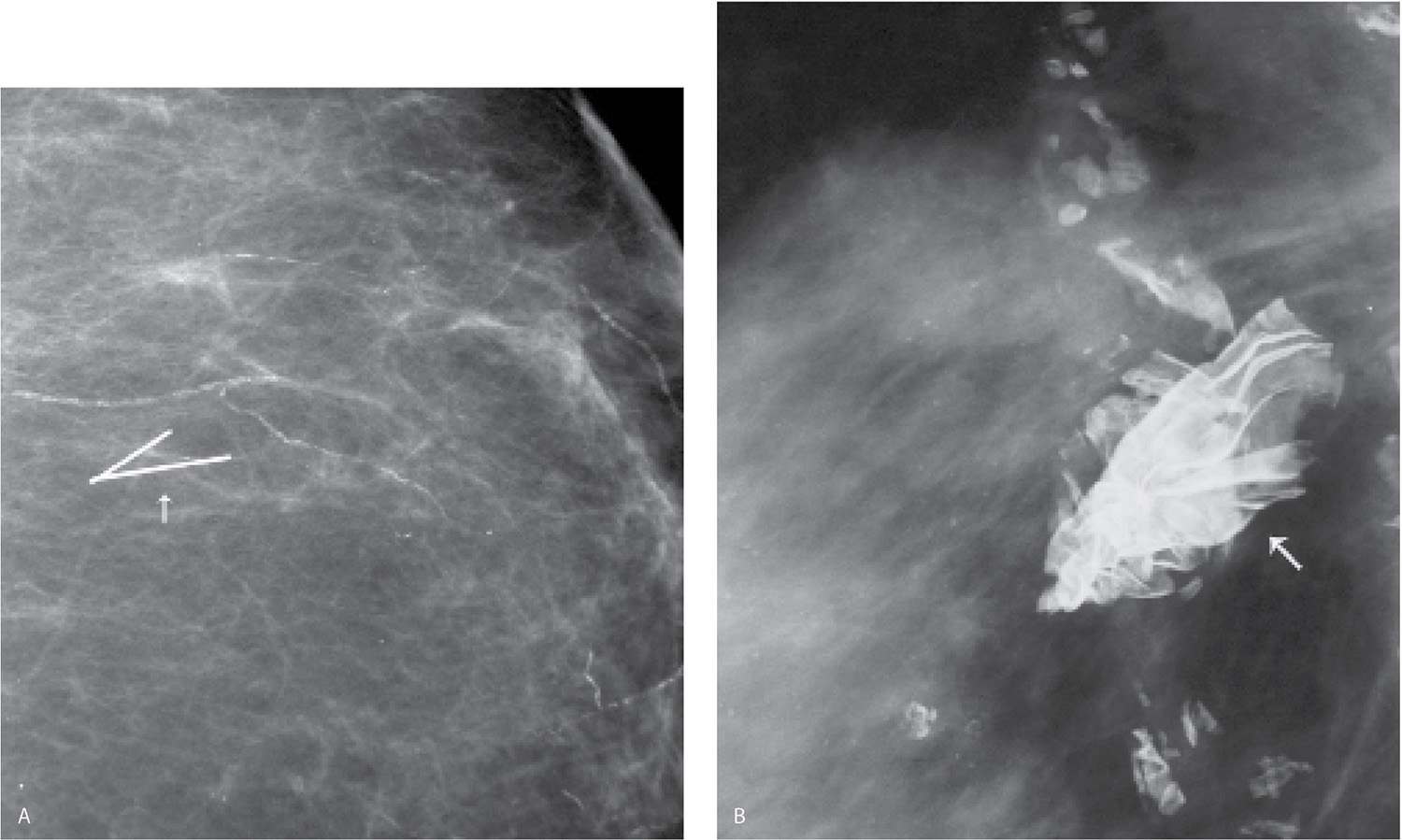
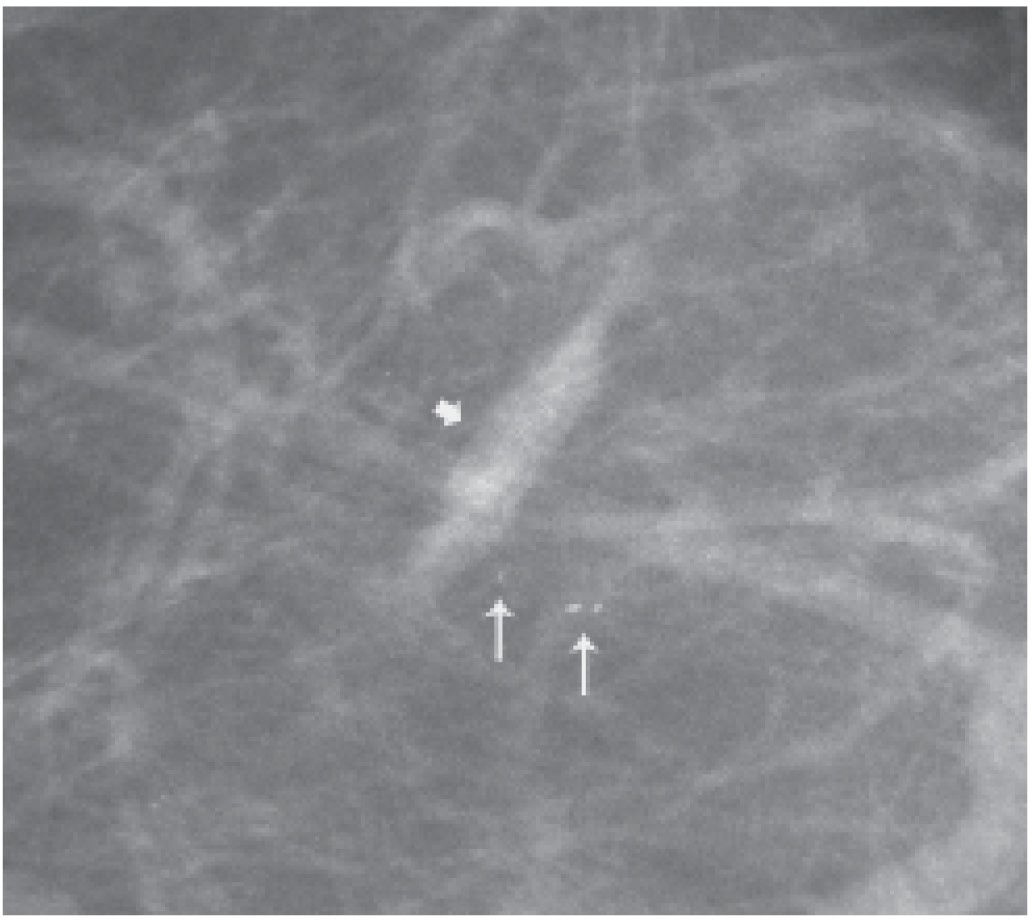
FIG. 11.6 • Retained localization wire fragment and foreign body. A: Intraoperative transection of the localization wire can result in retained wire fragments in the breast (arrow). Vascular calcification is present. B: Different patient. Radiopaque foreign body (arrow) is noted corresponding to a prior biopsy site. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)
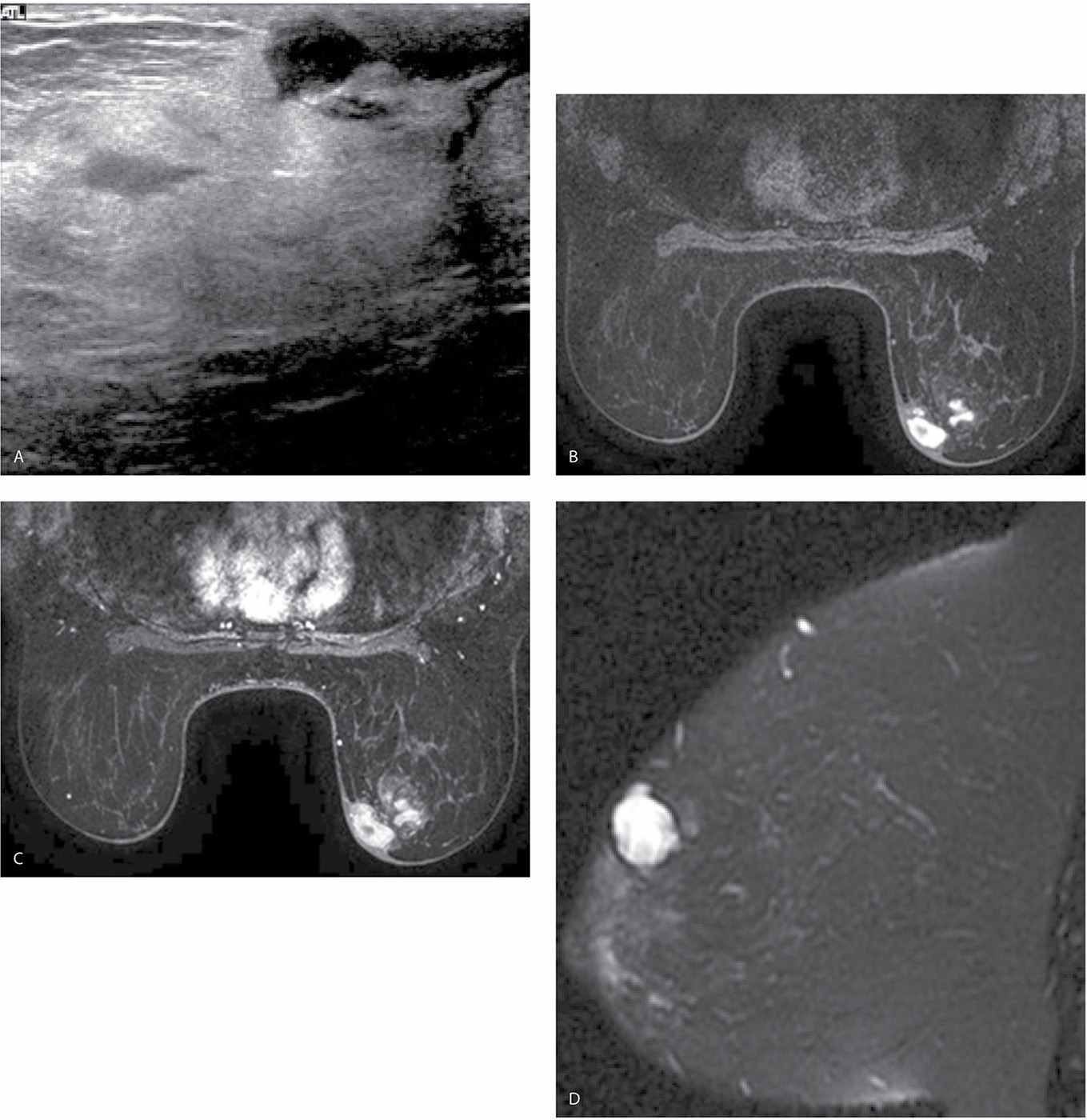
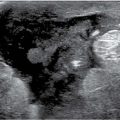
FIG. 11.7 • Acute hematoma right breast, after excisional biopsy. A: Ultrasound. An irregular fluid collection is imaged subcutaneously in the right subareolar area with increased echogenicity and loss of the normal tissue planes in the surrounding tissue. Normal soft tissue planes are apparent in the upper left corner of the image. This is a hematoma following an excisional biopsy. B: MRI, axial T1-weighted image pre-contrast. An irregular mass with circumscribed margins is present in the right subareolar area medially corresponding to the site of the excisional biopsy; irregular areas of internal high T1 signal are noted in the mass. C: MRI, T1-weighted image post contrast. No enhancement is noted in the mass. D: MRI T2-weighted sagittal fat-suppressed image of the right breast. Heterogeneously high T2 signal is noted in the right subareolar mass corresponding to areas of high T1 signal shown in part B consistent with an acute hematoma.
VACUUM-ASSISTED IMAGING-GUIDED BIOPSY
Vacuum-assisted, imaging-guided breast biopsies with an 11G (less commonly 14G) needle can result in complete removal of small lesions. In these patients, a radiopaque marker (often a titanium clip) is deployed to mark the site of the lesion. In patients having biopsies for MRI detected, mammographically occult lesions, the clip marks the site of the MRI detected abnormality for future reference. If a high-risk lesion or malignancy is diagnosed, the radiopaque marker is localized pre-operatively so that the high-risk lesion can be more completely characterized or wide excision of a malignancy can be accomplished. The clip usually remains at, or close to the biopsy site; however, migration of the clip can occur (6,7). If the patient does not undergo surgery, the radiopaque marker is seen on follow-up studies.
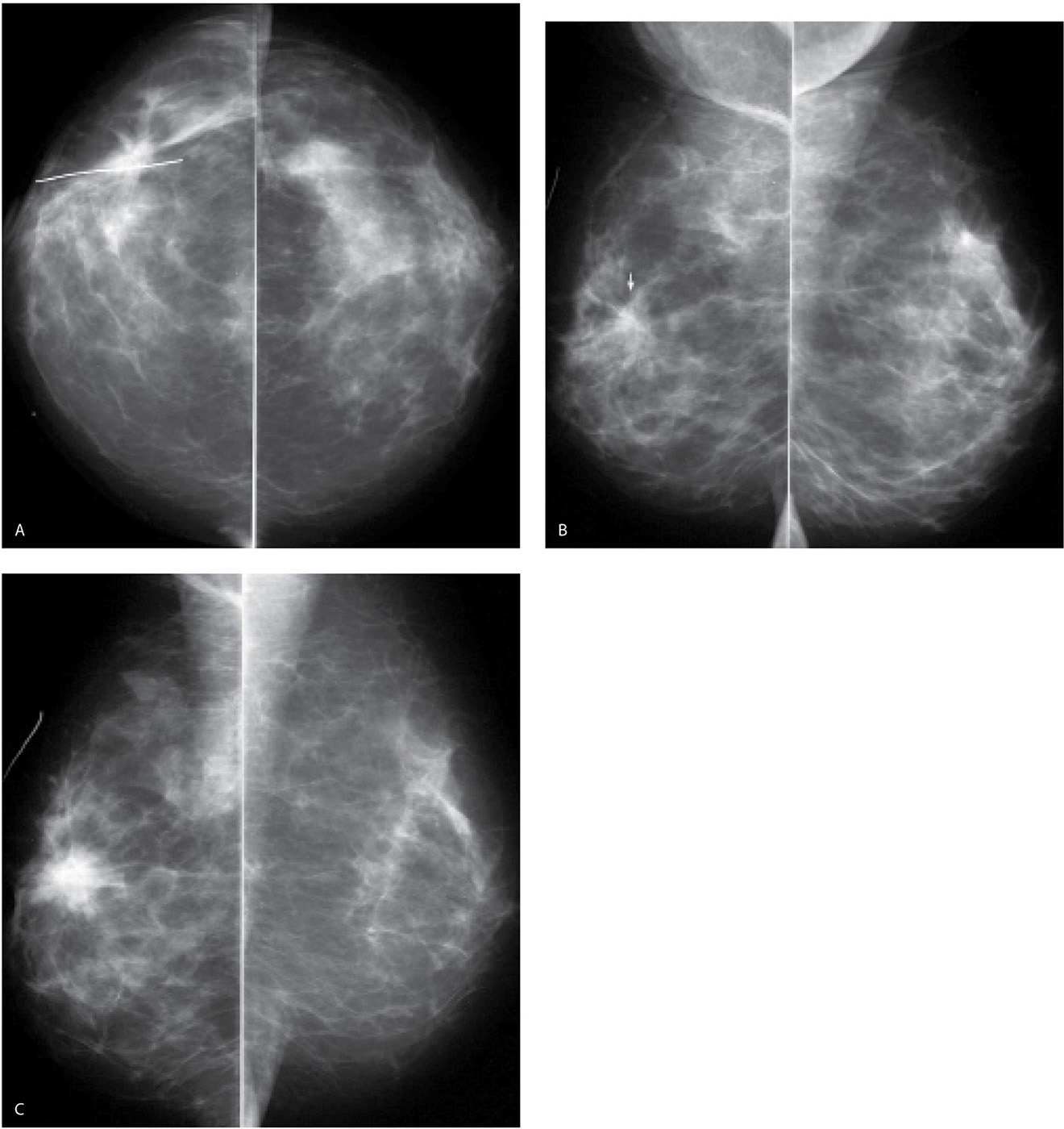
FIG. 11.8 • Invasive ductal carcinoma, not otherwise specified (NOS) occurring at prior biopsy site. CC (A) and MLO (B) views. A mass with spiculated margins and associated distortion is noted at a biopsy site (metallic wire) in the upper (arrow on MLO) outer quadrant of the right breast. Findings are attributed to the biopsy. Notice that at this point, the mass is more apparent on the CC projection. C: MLO views one year later. An irregular dense mass with spiculated margins and associated distortion is now apparent. The mass has increased in size and density compared with the prior study. Imaging-guided biopsy is done to establish the diagnosis of invasive ductal carcinoma. Apparent increases in density and distortion at a prior biopsy site warrant comparison with the earliest and all subsequent post-operative studies available. If the change is significant as in this patient, it may be appreciated from one year to the next; however, in many patients, the progression evolves slowly and may not be apparent from one year to the next.
If a clip is deployed during an imaging-guided biopsy, orthogonal images are done to document the accuracy of clip placement immediately after the biopsy procedure; we also comment if the original lesion is removed in its entirety. In addition to the radiopaque marker, an air locule or locules (Fig. 11.10) increased density in a tubular configuration (Fig. 11.11) in one of the images and more mass-like on the orthogonal view (e.g., denoting the needle track), and increased soft tissue stranding or a mass may be seen at the biopsy site (Fig. 11.12). If present, imaging-guided biopsy changes usually resolve within the first several days following the biopsy and do not produce long-term sequelae. If an MRI is done following an imaging-guided biopsy, fluid collections will usually have a high T2 signal, and the T1 signal will vary depending on the timing of the MRI with respect to the biopsy (in the acute setting they may have a high T1 signal that decreases with time); these are sometimes noted along the distribution of the needle track (Fig. 11.13).
FIG. 11.9 • Invasive ductal carcinoma, not otherwise specified, at prior biopsy site. A: Minor distortion is noted at a prior biopsy site (arrow). B: Three years following the image in part A. An irregular mass (arrow) with indistinct margins is apparent at the prior biopsy site. When comparison is made to the earliest study following the biopsy (image shown in part A), changes are easier to detect. Imaging-guided biopsy is done in this patient to establish the diagnosis. When evaluating distortion and densities associated with prior biopsy sites, be sure to look at the earliest post-operative study available, since slowly evolving changes may not be readily apparent from one year to the next. Benign coarse calcifications are seen in the adjacent tissue. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)
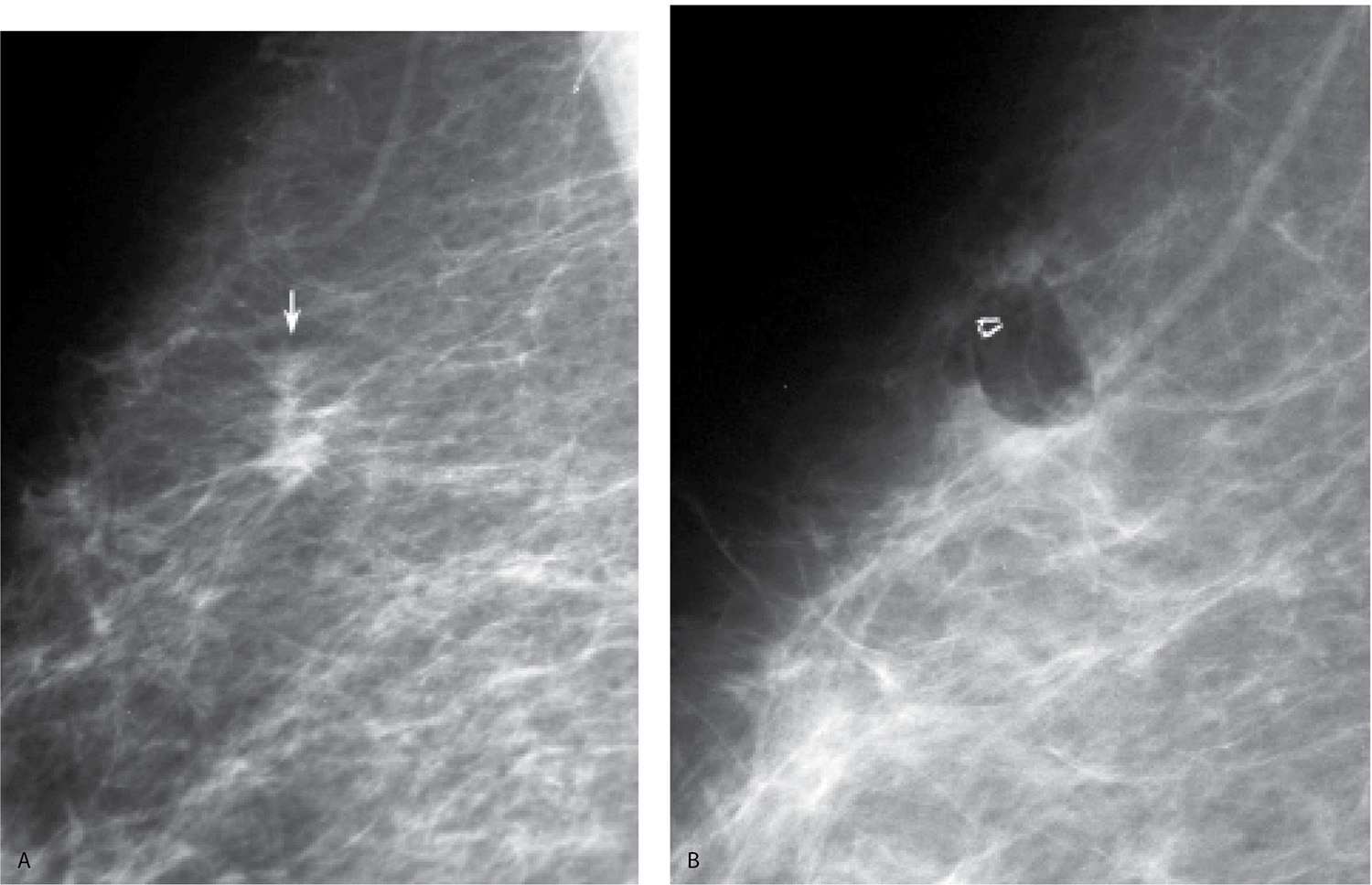
FIG. 11.10 • Invasive ductal carcinoma, NOS, clip post–vacuum-assisted breast biopsy. A: MLO view of the right breast photographically coned to the upper aspect of the breast. An irregular mass (arrow) with spiculated margins is identified superiorly in the right breast. B: Ninety-degree lateral (LM) view done after an 11G vacuum-assisted biopsy. The clip and an air locule are seen on orthogonal views (only one view is shown) at the biopsy site. A biopsy marker (in this patient a titanium micromark clip) is deployed to denote the location of the lesion and follow-up orthogonal images are done immediately after the biopsy to verify clip placement. As in this patient, with vacuum-assisted biopsies, small lesions or cluster of calcifications may be completed removed with the biopsy procedure. If cancer, or a high-risk lesion is diagnosed, and more tissue needs to be excised, the clip is localized and excised with the surrounding tissue on the day of the patient’s definite surgical procedure. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)
FIG. 11.11 • Hematoma, post–vacuum-assisted breast biopsy. Tubular area of increased density (thick arrow) is seen along the course of the 11G needle track. In the orthogonal view (not shown), this simulates a round mass. A clip is not deployed because calcifications (thin arrows) remain at the biopsy site following the procedure. DCIS is diagnosed on the core biopsy and confirmed on the lumpectomy specimen. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)
TRAUMA
Following trauma to the breast, patients may present acutely with an ecchymosis at the site of the trauma. Irregular areas of increased density, a mass reflecting the presence of a hematoma (Fig. 11.14A; also see Fig. 7.55), parenchymal asymmetry, or focal prominence of the trabecular markings (Fig. 11.15; also see Fig. 9.7) may be seen mammographically. On ultrasound, a hyperechoic or complex cystic and solid mass (Fig. 11.14B; also see Fig. 7.55B) may be seen alternatively; mass-like areas of hyperechogenicity with internal hypo- to anechoic areas are common sonographically. Clinically, the ecchymosis resolves. Mammographic and sonographic findings also usually resolve; however, some patients develop a hard palpable mass on physical exam that is often a fat-containing mass mammographically (Figs. 11.14D, 11.16A, and 11.17A). Alternatively, an oil cyst and dystrophic calcifications may develop at the site of trauma (Fig. 11.16B). Many patients do not present acutely following the trauma but rather months after the incident. Consequently, if a fat-containing mass is seen mammographically with hyperechogenicity and internal hypoechogenicity on ultrasound (Fig. 11.17B), specifically ask the patient about trauma. Also, before assuming a mammographic finding is related to trauma, consider the location of the lesion; trauma is typically going to involve the upper quadrants of the breasts, and it is unlikely to involve the lower central aspects of the breasts posteriorly. In some patients on anti-coagulants, hematomas may develop with the patient having no recollection of significant trauma (Fig. 11.17).
Following a seat belt injury, patients may present with a band-like area of increased density corresponding to the course of the seat belt. The findings are localized to the upper inner or central quadrants of the left breast or the lower inner quadrant of the right breast when the patient is the driver. Findings in the upper inner quadrant of the right breast (Fig. 11.18) or lower inner quadrant of the left breast may be identified when the patient is the front seat passenger (8).
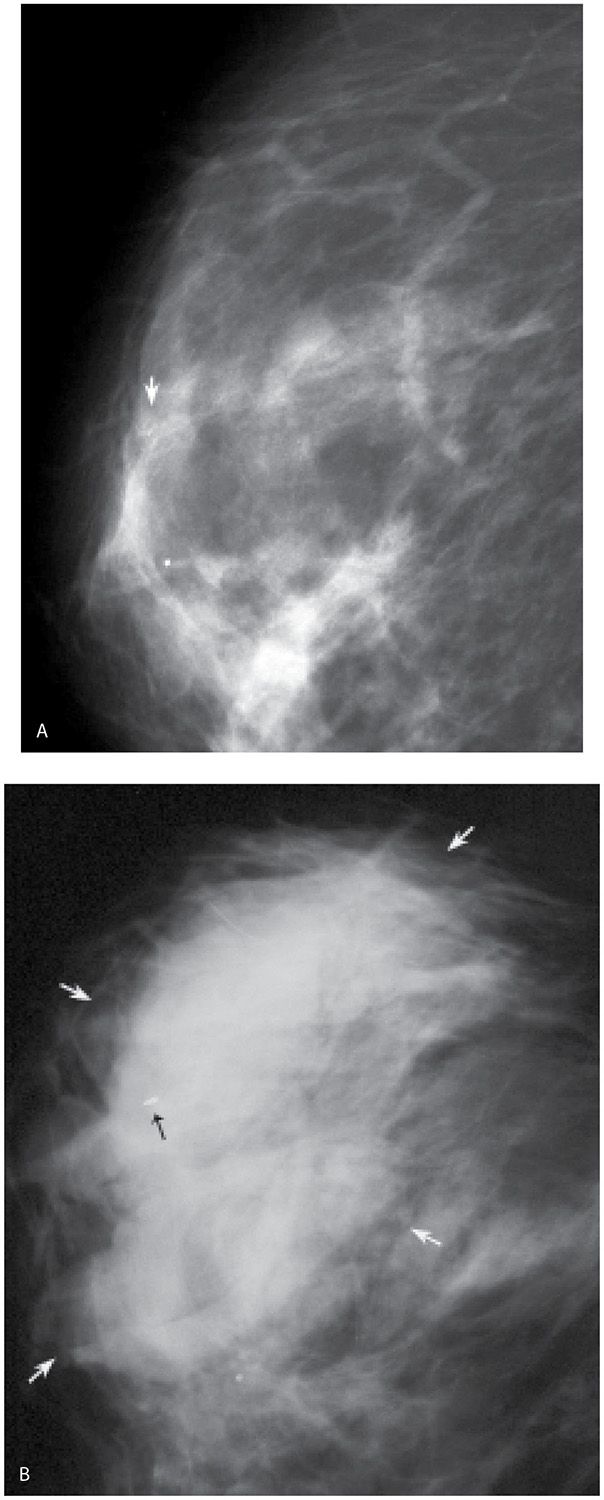
FIG. 11.12 • Hematoma, post–vacuum-assisted breast biopsy. A: MLO view, right breast pre-biopsy. Grouped fine pleomorphic calcifications (arrow). B: Micromark clip is deployed (black arrow) at the site of the vacuum-assisted biopsy (11G) for calcifications (benign changes described histologically). Increased density (white arrows) is noted related to the presence of a hematoma. Although not common, hematomas can occur following imaging-guided breast biopsies. These typically resolve spontaneously. Rarely, surgical evacuation is needed. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)
FIG. 11.13 • Invasive ductal carcinoma, NOS, needle track post-biopsy. A: MRI, sagittal T2-weighted fat-suppressed image of the right breast. A mass (long arrow) with a low T2 signal is imaged upper aspect of the right breast posteriorly. Multiple smaller masses (short arrows) characterized by a high T2 signal extend linearly from the skin to mass denoting the needle track formed during an ultrasound-guided 14G biopsy of the mass. Edematous changes (high T2 signal) are noted in the surrounding tissue. B: MRI, sagittal T1-weighted reconstruction of the right, post contrast. Oval mass (long arrow) with heterogeneous enhancement and irregular margins is noted correlating to the mass seen on the T2 image shown in part A and the site of the patient’s known malignancy. Non-enhancing soft tissue stranding is noted along the needle track (short arrows); the masses with high T2 signal noted in part A have a low T1 signal and demonstrate minimal peripheral enhancement. Note skin thickening and enhancement corresponding to the skin entry site for the biopsy.
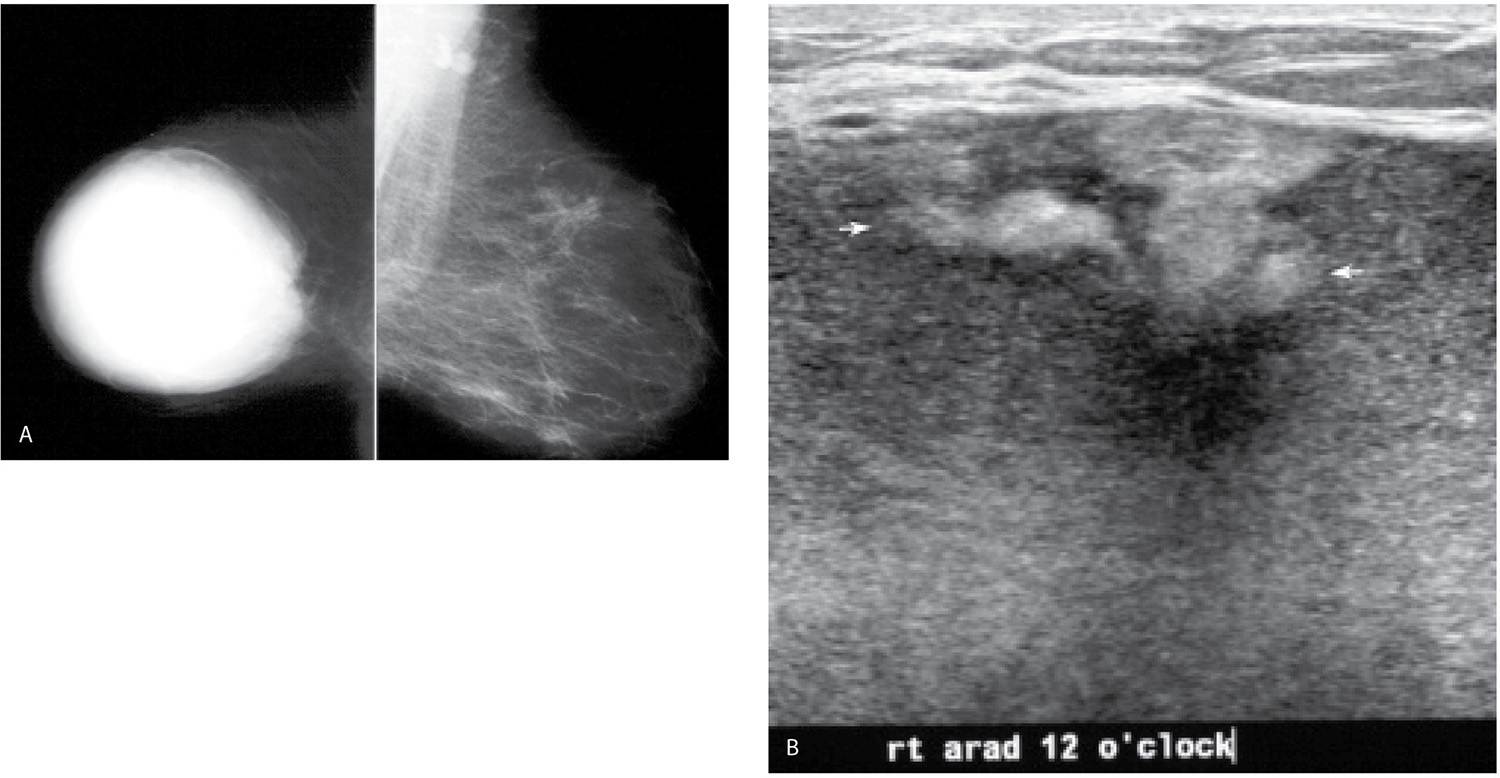
FIG. 11.14 • Hematoma, evolution. A: MLO views. Acutely following trauma, a round dense mass with circumscribed margins is present almost completely involving the right breast. In an attempt to penetrate through the mass, the remainder of the breast tissue is “burned” out. Technical factors for the exposure of the right breast: compression = 9.9 cm, kVp = 35, mAs = 400. B: Ultrasound. Given the size of this mass, it is imaged in parts. This image is a fair representation of the lesion with cystic areas and hyperechoic mural nodules (arrows). C: MLO views. A round dense mass with circumscribed margins persists 2 years after the accident; however, it has decreased in size. D: MLO views 5 years after the accident. Multiple low-density and fat-containing masses with circumscribed margins are now seen at the site of the original hematoma.
CONSERVATIVE BREAST CANCER TREATMENT
The primary aim of breast conserving treatment is adequate local control of breast cancer. Wide surgical margins are desired in minimizing local recurrences. The cosmetic results, however, are an important secondary consideration. If a substantial amount of tissue needs to be removed to obtain clear margins in a patient with a small breast, cosmesis may not be acceptable. Radiation therapy is used to control any residual occult breast cancer. It is begun 2 to 5 weeks after the lumpectomy. Treatment is given 5 days a week for a total of 5 weeks and delivers 45 to 50 Gy to the affected breast. A boost to the lumpectomy site may be given using an electron beam or iridium implant, increasing the total dose delivered to 60 to 66 Gy (2). Although not all patients with breast cancer are good candidates, high doses of radiation therapy delivered over a 5-day period to the site of the tumor (brachytherapy) are being used with early results comparable to those of whole breast radiation. The other alternative being used with increasing frequency is neoadjuvant therapy as the initial treatment. This approach has been traditionally reserved for patients with inflammatory carcinoma; however, it is now being extended to include two other groups of patients: (i) those with known metastatic disease to the axilla in whom systemic disease is a concern and (ii) those with larger primary lesions in whom conservative therapy is not a good option at the time of presentation but for whom it can become an option if the tumor shrinks (see Chapter 5 for additional discussion regarding imaging in these patients).
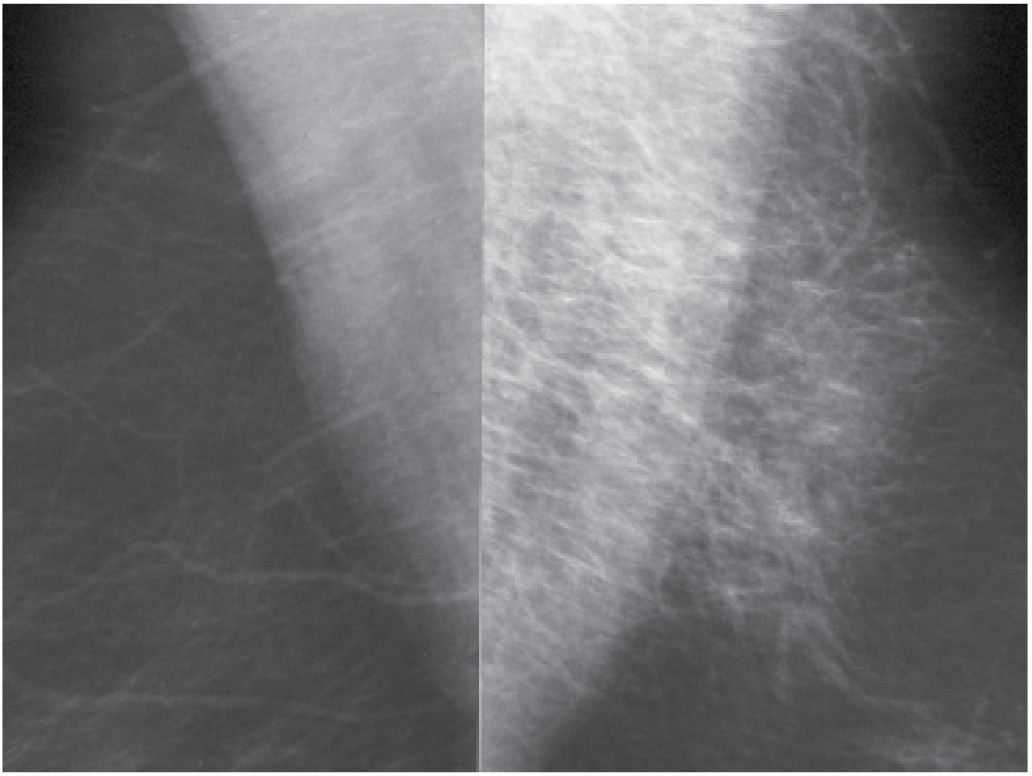
FIG. 11.15 • Hematoma. MLO views photographically coned to the upper portion of the images. Thickening and increased density of the trabecular markings is seen corresponding to the site of an ecchymosis in the upper portion of left breast following trauma to the breast. (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.)
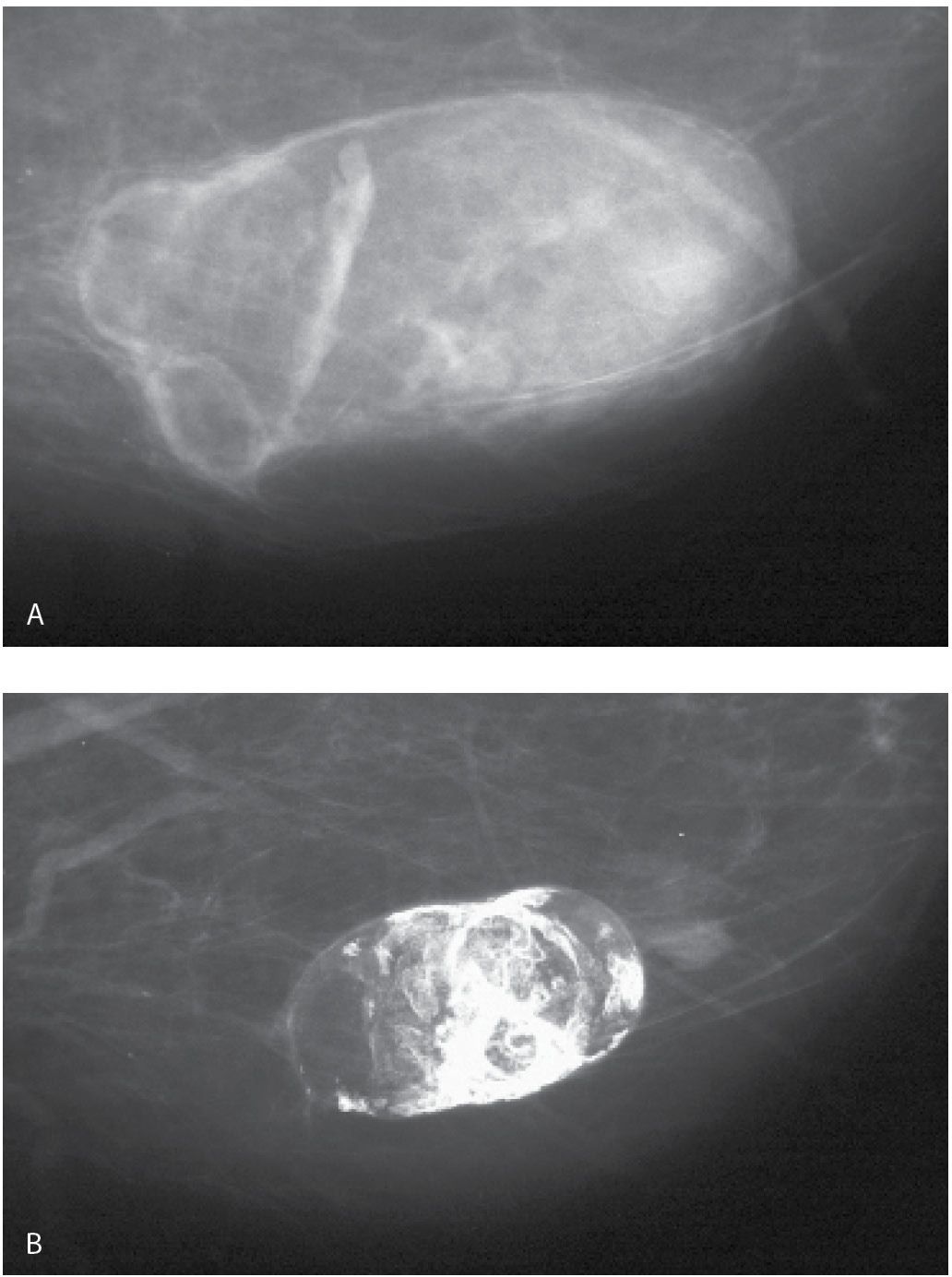
FIG. 11.16 • Hematoma, evolution. A: Oval fat-containing mass is imaged corresponding to a site of trauma in the left breast. B: Seven years later, the mass is smaller with dense dystrophic mural calcifications (From Cardeñosa G. Breast Imaging [The Core Curriculum Series]. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.).
FIG. 11.17 • Hematoma. A: Spot tangential view done at the site of “lump” described by the patient (metallic BB denotes palpable finding). Fat-containging (e.g. mixed density) mass with indistinct margins and internal halo is imaged at the site of the palpable finding. B: Ultrasound, transverse (TRV) plane. On physical examination a healing ecchymotic area is apparent on the skin overlying the palpable finding. The patient states she is on Coumadin and bruises easily. An oval mass with hyperechoic and anechoic components (heterogeneous echotexture) is imaged corresponding to the palpable and mammographic finding in the left breast. BI-RADS 2: Benign finding.
Mammographic changes related to whole breast radiation therapy involve the breast diffusely and are primarily related to edema. These include skin and trabecular thickening resulting in increases in parenchymal density and reduced breast compressibility. The changes usually resolve within the first 2 years following treatment (Fig. 11.19; also see Fig. 9.1). Technical factors for adequate exposure of the remaining breast tissue may need to be adjusted accordingly. In the acute setting, skin thickening, dilated lymphatics, and increased tissue echogenicity may be seen on ultrasound following radiation therapy. Residual skin thickening is seen in approximately 20% of women 2 years after radiation therapy (2). Rarely, patients develop fairly extensive fat necrosis that involves the breast diffusely (e.g., not localized to the lumpectomy site) with progressive fibrosis, contracture, and patient discomfort (Fig. 11.20). It is unclear if this process is related solely to the radiation or if it reflects a combination of radiation therapy and surgical effect. Long-term complications of radiation therapy are now also being seen with increasing frequency in part a function of an increase in the number of follow-up years in women who have had whole breast radiation therapy for breast cancer. Patients may present with pulmonary fibrosis, pleural effusions, rib fractures (osteonecrosis), and secondary malignancies often sarcomatous in nature involving the skin (e.g., cutaneous angiosarcomas), breast parenchyma (Fig. 11.21), or soft tissues of the chest and upper abdominal wall (Fig. 11.22) included in, or surrounding, the radiation field (9,10).
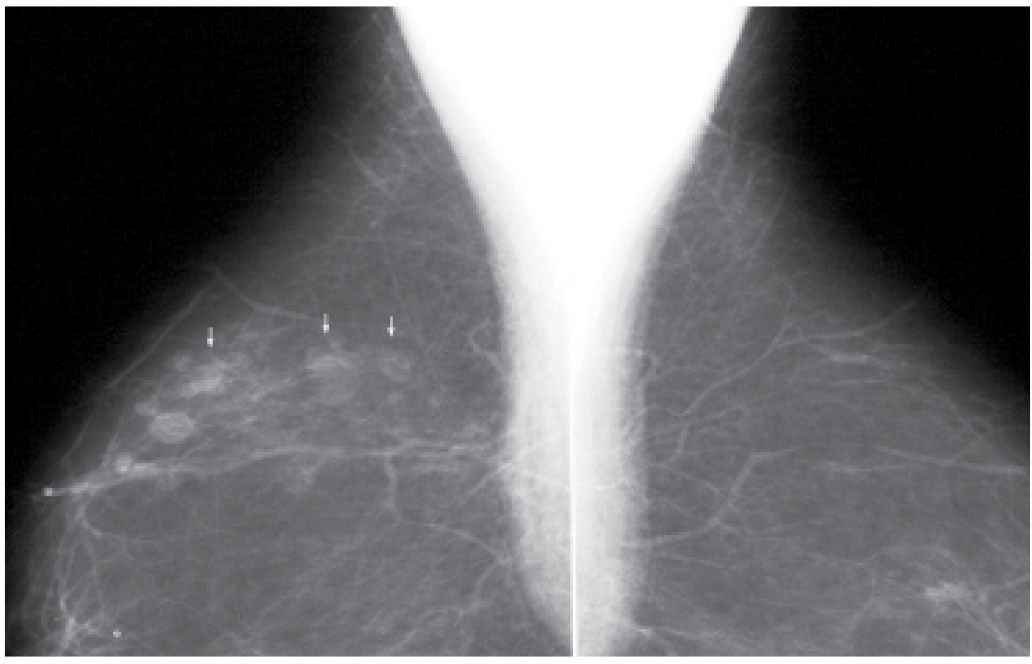
FIG. 11.18 • Seat belt injury, oil cysts. MLO views. Linearly distributed oil cysts in a patient following a car accident several years previously.
The findings seen mammographically following lumpectomy are variable, usually evolve with time and are localized to the surgical site (Fig. 11.19). These include irregular increases in density, distortion, a mass with spiculated margins (fat necrosis), and a mass at the lumpectomy site reflecting a post-operative fluid collection as well as localized skin thickening and retraction. On MRI, non-enhancing distortion and a non-enhancing mass with spiculated margins are the most common findings noted at prior lumpectomy sites; associated skin thickening and retraction are also seen and, in some patients, depending on the location of the original tumor, tenting of the pectoral muscle.
Fat necrosis developing at the lumpectomy site is variably sized and characterized by an irregular mass with indistinct, or more commonly spiculated margins, or distortion alone (Fig. 11.19C, D). As the acute inflammatory changes associated with fat necrosis resolve, the mass or area of distortion decreases in size and density, and oil cysts or dystrophic calcifications (Fig. 11.23) can be seen developing at the lumpectomy site (2,4,11–17). On ultrasound, fat necrosis often results in disruption of normal soft tissue planes as well as distortion and intense shadowing at the surgical site sometimes associated with an irregular mass (5).
Fluid collections are seen in as many as 50% of patients within the first 4 weeks after the lumpectomy (2). An oval or round (Figs. 11.19A, B and 11.24A) mass with circumscribed to indistinct or spiculated margins (Fig. 11.24C) is identified at the lumpectomy site. These are variable in density; however, some are relatively low in density particularly when considering their size. They may have lucent locules or fluid–fluid levels. An internal halo may be present partially outlining the inner margin of the mass (Fig. 11.25). On ultrasound, complex cystic and solid masses with septations (Fig. 11.26A; also see Fig. 4.29B), thickened walls, or echogenic nodules (Fig. 11.26B; also see Fig. 4.29A) are imaged corresponding to the masses seen mammographically. Less commonly a complex cystic and solid mass that is predominantly solid with cystic spaces (Fig. 11.24B and 11.26C) or a nearly anechoic mass is seen. Most fluid collections resolve within the first 2 years after the lumpectomy; however, some may persist for years. If the patient is asymptomatic, aspiration is not indicated and should be avoided because, in many patients, the fluid re-accumulates rapidly after percutaneous drainage, the re-accumulated fluid collection may be larger than the starting point and chronic draining sinuses can develop (Fig. 11.27; also see Fig. 4.30). Draining sinuses can be hard to manage and affect the patient’s quality of life significantly. The signal characteristics of post-operative fluid collections on MRI are variable depending on the age of the collection. Acutely, they may demonstrate high T1 and T2 signal with minimal rim enhancement. As the collection ages, it decreases in size, the margins become better defined and the T1 signal usually decreases. Fluid–fluid levels may be apparent (Fig. 11.28).
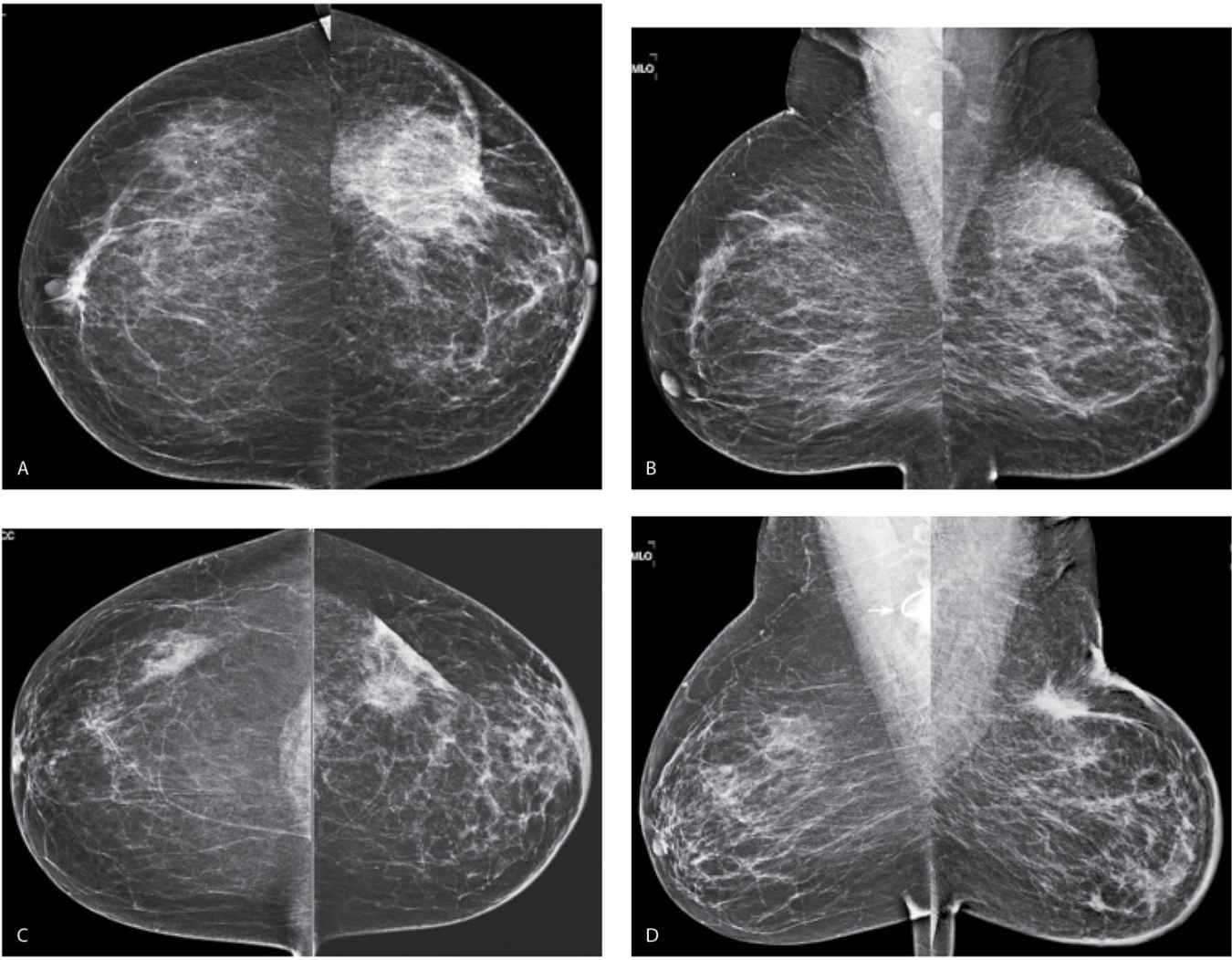
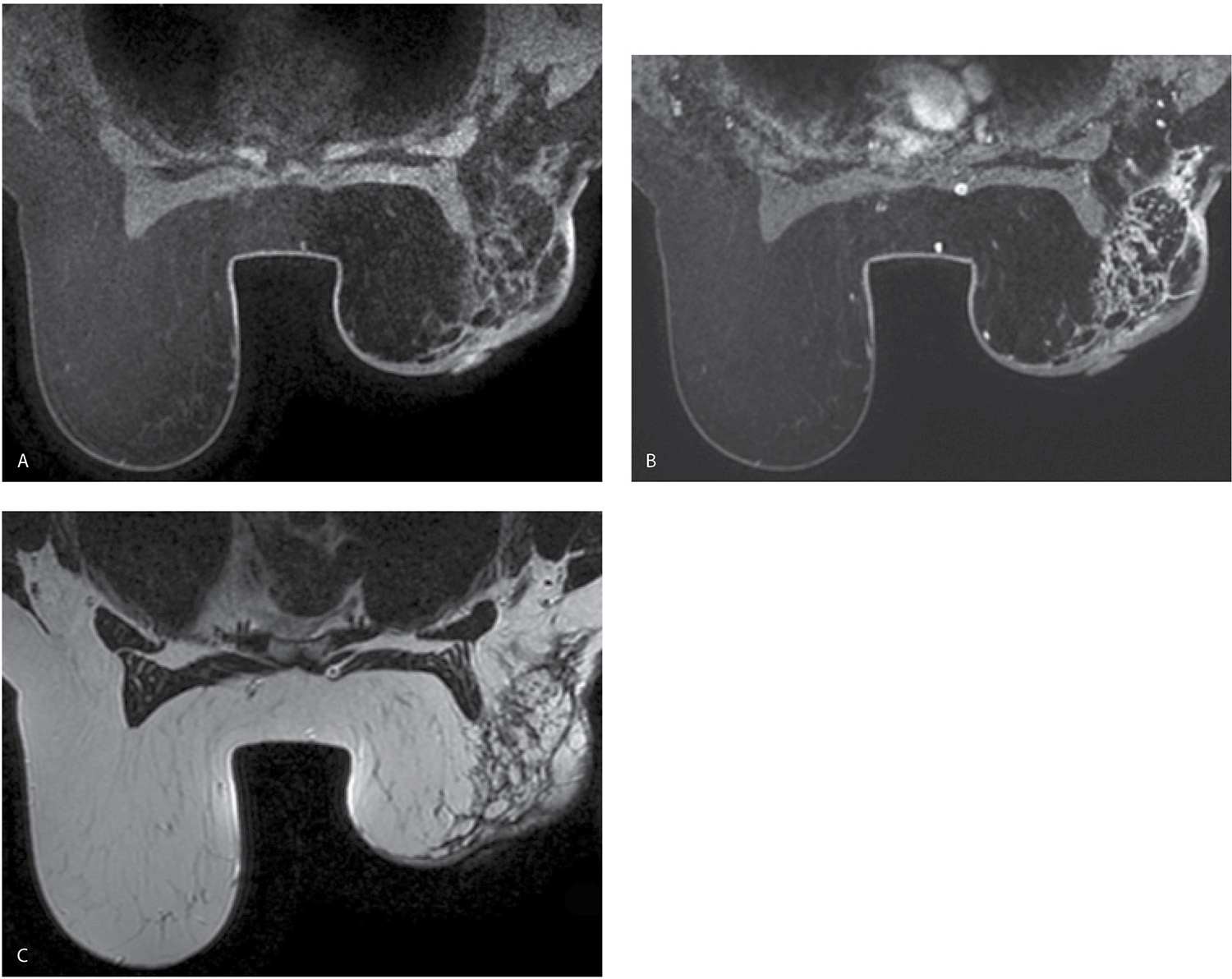
FIG. 11.19 • Lumpectomy and radiation therapy changes. CC (A) and MLO (B) views done in a 55-year-old patient. First study post lumpectomy and radiation therapy for DCIS in the left breast. The left breast is smaller than the right. Diffuse skin thickening as well as increased and thickened trabecular markings reflecting edematous changes related to the radiation therapy are noted involving the left breast. A round low-density mass with indistinct margins is imaged in the upper outer quadrant of the left breast consistent with a fluid collection at the lumpectomy site. Thickening and retraction of the skin are noted localized to the lumpectomy site. Compare the technical factors used for exposure on the right CC view: compression = 55 mm, kVp = 25, mAs = 165 with those used for the left CC: compression = 66 mm, kVp = 27, mAs = 236; the difference in compression reflect decreased compressibility of the radiated breast and the needed increase in kVp and mAs are related primarily to skin thickening. CC (C) and MLO (D) views in a 65-year-old patient. First study post lumpectomy and radiation therapy for a high-grade invasive ductal carcinoma in the left breast. The left breast is smaller than the right. Diffuse skin thickening as well as increased and thickened trabecular markings reflecting edematous changes related to the radiation therapy are noted involving the left breast. A mass with spiculated margins is imaged in the upper outer quadrant of the left breast associated with localized skin thickening and retraction. Note the appearance (shape and density) of the mass between the MLO and the CC views. One of the imaging features of fat necrosis at lumpectomy sites is a differential appearance between the routine views: it is often more apparent in one projection (in this patient the MLO) compared with the other (CC). Compare the technical factors used for exposure on the right CC view: compression = 49 mm, kVp = 24, mAs = 165 with those used for exposure on the left CC: compression = 58 mm, kVp = 26, mAs = 185. A round density and a tube (arrow) are partially imaged in the right axilla related to the presence of a central venous access catheter.
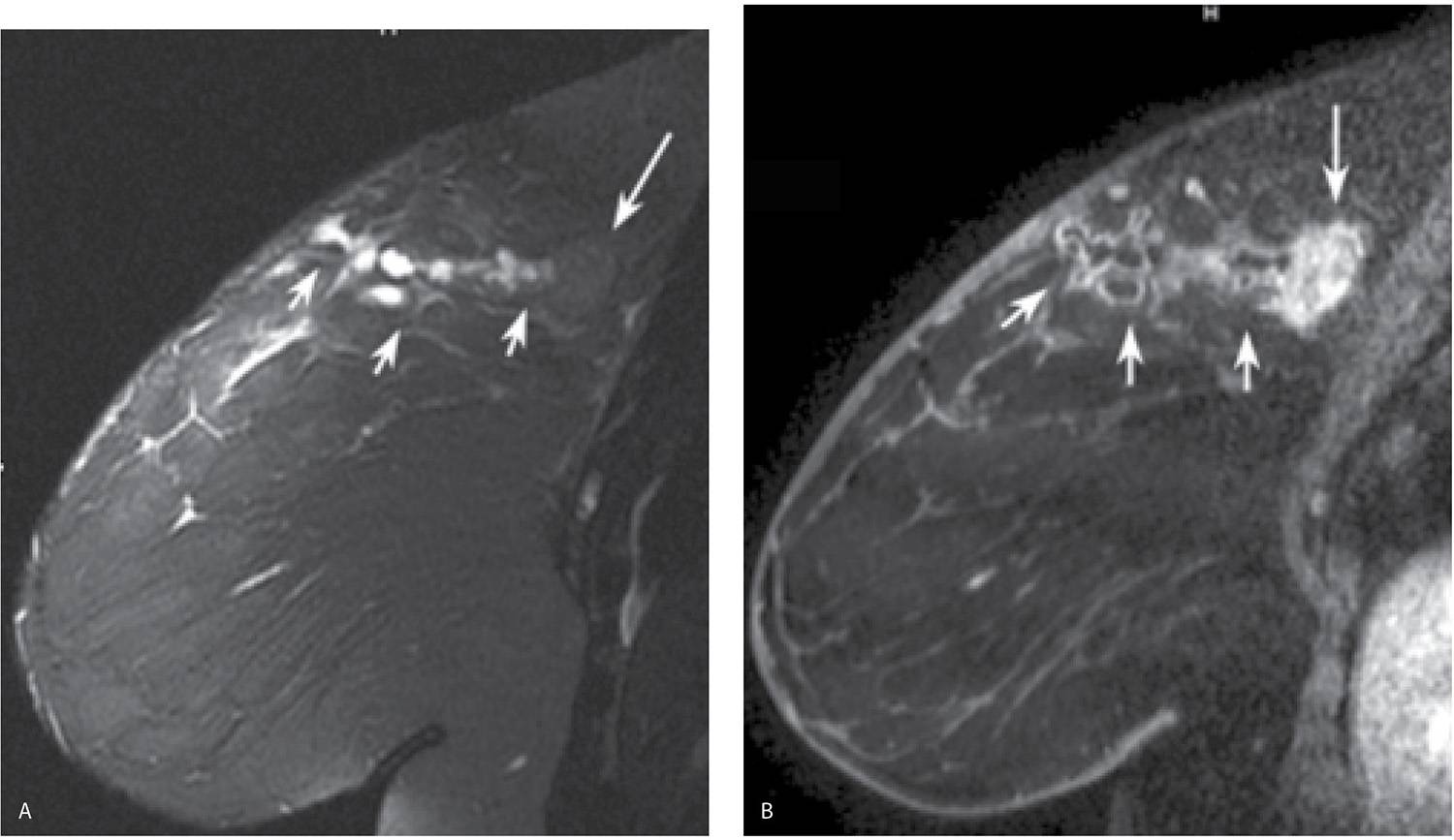
FIG. 11.20 • Fat necrosis. MRI, axial T1-weighted image pre contrast (A), MRI axial T1-weighted image, post contrast (B) and MRI, axial T2-weighted image (C) all at the same scan position, in a 57-year-old patient 2 years following lumpectomy and radiation therapy who presents describing a hard mass in the right breast with significant associated pain and discomfort (unable to tolerate a mammogram). The right breast is smaller and deformed compared with the left. An irregular mass with a thin rim and internal septations that enhance, irregular margins and internal fat is imaged encompassing the lateral quadrants of the right breast. The findings are consistent with fat necrosis following lumpectomy and radiation therapy.
Currently, there is no consensus on an appropriate follow-up protocol for patients following lumpectomy. If the patient is asymptomatic following conservative breast cancer therapy, the American College of Radiology practice guidelines on screening and diagnostic mammography (18) leaves the decision on how to schedule (e.g., as screening or diagnostic studies) these patients at the discretion of the imaging facility. Some facilities recommend 6-month follow ups of the treated breast for 3, 5, or 7 years and annual imaging of the contralateral, presumably normal breast (2). In our practice, we obtain a pre-radiation mammogram on patients who presented with an extensive area of calcifications. Rarely, we identify patients with residual calcifications at the lumpectomy site (14) who may benefit from re-excision before radiation therapy is started. Following completion of radiation therapy, we schedule patients for diagnostic studies annually for 7 years after which we return them to screening. We are not routinely obtaining 6-month follow ups of the treated breast following conservative therapy since there is nothing to suggest that recurrences grow any faster than primary lesions. In addition to routine views (CC and MLO), we obtain a spot compression magnification view of the lumpectomy site in tangent to the x-ray beam.
When evaluating these patients, it is helpful to have information on those features of the initial tumor that may influence the likelihood of recurrence including tumor size and grade, proximity of tumor to margins, presence of an extensive intraductal component, lymphovascular space involvement, and lymph node status. Additionally, it is helpful to know if the patient had radiation, chemotherapy, and if she is being treated with tamoxifen. Ideally, the patient’s imaging evaluation at the time of diagnosis is available for review since recurrences often resemble the appearance of the primary. The likelihood of recurrence is low in the first 2 years following treatment; it may be that some of the lesions identified within this time period reflect residual (inadequately treated) disease and not necessarily a recurrence. In the first 7 years following treatment, recurrences are likely to arise at or close to the lumpectomy site (Figs. 11.29 and 11.30); after this, recurrences or second primaries develop with an equal frequency anywhere in the breast (Fig. 11.30) (19–21). Fine pleomorphic calcifications with linear forms or demonstrating linear distribution, developing at prior lumpectomy sites should be biopsied (Fig. 11.29). Developing masses (Fig. 11.31) or increases in the size and density of architectural distortion at the lumpectomy site may also indicate recurrence. Rarely, recurrences will present with diffuse changes (Fig. 11.32; also see Fig. 9.17). In addition to meticulously evaluating the treated breast, carefully evaluate the contralateral side, since patients with a personal history of breast cancer are at increased risk for the development of breast cancer in the contralateral side (Fig. 11.33; also see Figs. 2.47 and 8.18). Aggressively pursue developing changes with spot compression or spot compresson magnification views, correlative physical examination and ultrasound.
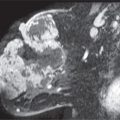
FIG. 11.21 • Radiation induced osteosarcoma. A: MLO views in a 64-year-old woman 8 years following lumpectomy and radiation therapy for invasive ductal carcinoma in the left breast. The left breast is smaller. An oval mass (short arrows) with two components, one dense (anteriorly) and the other isodense is identified in the upper outer quadrant of the left breast; this is new compared with the prior study. A metallic clip (long arrow) with surrounding distortion is seen inferiorly denoting the prior lumpectomy site. B: Ultrasound. A mass with a thickened echogenic curvilinear component and intense shadowing as well as circumscribed margins superficially and indistinct margins laterally is imaged corresponding to the dense portion of the mass. A giant cell neoplasm (metaplastic or giant cell) is reported on the ultrasound-guided core specimens. C: MRI sagittal T2-weighted image of the left breast, fat suppressed. A mass with internal high T2 signal and intermediate T2 signal peripherally is imaged corresponding to the mammographic and ultrasound finding. Also note the sharp demarcation between the round (short arrow) component anteriorly (dense mammographically) and the more posterior portion (long arrow) of the mass. Edematous changes are noted as “wispy” high T2 signal in the surrounding tissue extending to the pectoral fascia. D: MRI, T1-weighted axial image of the left breast, post contrast. Bilobed mass, the anterior portion of which demonstrates thickened irregular rim enhancement with rapid wash-in and wash-out delayed kinetics. Low internal T1 signal correlates to the high T2 signal seen in the mass, part D consistent with internal necrosis. An osteosarcoma, presumably radiation induced, is diagnosed following a left mastectomy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree