Johny A. Verschakelen, Fergus Gleeson
The Chest Wall, Pleura, Diaphragm and Intervention
The Chest Wall
Although there are a wide variety of tissues and structures that make up the chest wall, based on their radiographic presentation, its components can be grouped into two major parts: the soft tissues and the bony structures.
Soft Tissues
On the chest radiograph the soft tissues present as areas of increased density that in part project next to the bony chest wall and in part overlay the different components of the chest. Abnormalities of the soft tissues will present as an abnormal increase or decrease in density often combined with the appearance of an abnormal contour or the disappearance of a normal contour.
Because of better density resolution and multiplanar reformatting, computed tomography (CT) can better demonstrate the different tissues of the chest wall. CT has the advantage over magnetic resonance imaging (MRI) of higher spatial resolution and the ability to better identify bony structures. MRI, however, yields greater soft-tissue contrast, which can be important.1,2 Multiplanar imaging and three-dimensional (3D) reformation can be performed with both techniques.
Ultrasound may also be used to examine the chest wall. In general it provides less detailed and comprehensive information but it usually enables the lesion to be localised, allows a distinction to be made between cystic and solid lesions and enables guided aspiration/biopsy to be performed under imaging control.
Breasts
On the female chest radiograph it is mandatory to check that both breasts are present. Unilateral radical mastectomy is usually easy to detect because it generates a unilateral mid/lower zone transradiancy and an abnormally straight anterior axillary fold that passes upwards and inwards towards the mid clavicle (Fig. 10-1). Bilateral radical mastectomy is more difficult to identify, but an overall increase in basal transradiancy and axillary fold abnormalities should provide adequate clues. Surgical interventions short of radical mastectomy may be impossible to detect, but close attention to the relative transradiancy of the breast regions and to the breast contours may provide suggestive findings. In addition, the presence of vascular clips can be helpful.
Nipple shadows can mimic intrapulmonary nodules. A putative nipple should be checked for compatible size (5–15 mm), shape and location—its relation to the breast outline in a woman or the pectoralis opacity in a man.
Muscles
On the chest radiograph the pectoralis major produces a broad, band-like opacity extending downwards and medially from the axilla. Unilateral absence or hypoplasia of the pectoralis major results in a unilateral transradiancy and an abnormal anterior axillary fold as seen with mastectomy. In Poland’s syndrome these changes are accompanied by ipsilateral hand and arm anomalies (particularly syndactyly) with or without absence of pectoralis minor, rib anomalies and hypoplasia of breast and nipple.
Soft-Tissue Calcification
Soft-tissue calcification may occur in the chest wall, and clues to its site and nature are provided by its morphology distribution and the clinical history. Possible causes to consider include granulomatous lymph nodes, parasites (Taenia solium and Dracunculus medinensis), calcinosis universalis, childhood dermatomyositis, tuberculosis (spine, ribs or soft tissues) and bone neoplasms. Ossification is rare and most commonly seen in fibrodysplasia ossificans progressiva.
Subcutaneous Emphysema
Subcutaneous emphysema of the chest wall is not uncommon following surgery, pleural drain placement, trauma or in cases of spontaneous or acquired pneumomediastinum. Air dissects along tissue planes and between muscle bundles, giving an overall pattern of linear transradiancies which can significantly interfere with the interpretation of the underlying structures. In this way diagnosis of pneumothorax can become very difficult. In case of doubt, CT can be performed.
Soft-Tissue Tumours
A soft-tissue tumour of the chest wall gives rise to an opacity. Malignant and inflammatory lesions cause bony destruction and benign ones result in rib separation and notch-like remodelling from pressure erosion.
The most common benign chest wall tumour is a lipoma, but a variety of other mesenchymal tumours occur, including neurofibromas (focal or plexiform), neurilemmomas, haemangiomas and lymphangiomas (cystic hygromas). On CT, lipomas are well-demarcated homogeneous masses of low density (–90 to –150 HU). They contain few, if any, other soft-tissue components; the presence of the latter in a fatty tumour suggests a liposarcoma. MRI features are also characteristic, with high signal on T1-weighted images, intermediate signal on T2-weighted images and low signal with fat suppression.1,2 Neurofibromas on CT characteristically have a lower density than muscle both before and after intravenous contrast medium. On MRI, neurofibromas give low-to-intermediate signal on T1-weighted images but high signal on T2-weighted images and marked contrast enhancement after gadolinium, which allows clear delineation of their extent. Haemangiomas are uncommon lesions that occasionally show phlebolithic calcification on plain radiography. Findings on CT include phleboliths, bone remodelling and an enhancing mass. MRI is the best investigation for delineating their extent. Lesions give an intermediate signal on T1-weighted images and a high signal on T2-weighted images, accompanied by artefacts generated by vessels, soft tissue and elements derived from haemorrhage.1,2 Lymphangiomas on CT have the features of a fluid-filled cyst with or without septation. On MRI they have the features of a cyst with low protein content.
Malignant primary tumours arising in the soft tissues of the chest wall are unusual, the most common being lipo- or fibrosarcomas (Fig. 10-2).
Secondary tumours of the chest wall are common, particularly when due to local spread (carcinoma of the breast and lung, lymphoma); see bronchial carcinoma and Pancoast’s tumour, below.
Bony Structures
Although depicting bone abnormalities is not the primary goal of a chest radiograph, the bony structures that are, as a result of the technique, often only partially visible should be carefully examined. CT, when indicated, is better at demonstrating congenital or acquired remodelling or complicated fractures; multidetector 2D or 3D reformations can be helpful.
Ribs
There are normally 12 pairs of ribs. Cervical ribs occur in 1–2% of the population and are commonly bilateral, though often asymmetrical.
Congenital abnormalities of modelling may be confined to one or two ribs or be generalised. One or a few upper ribs are commonly bifid, splayed, fused or hypoplastic. Usually occurring in isolation, these anomalies are occasionally part of a syndrome (e.g. basal cell naevus syndrome) or associated with other anomalies (e.g. Sprengel’s deformity).
With acquired remodelling, abnormalities tend to be focal, affecting one or many ribs. Such acquired changes may follow fracture, surgery, osteomyelitis and empyema drainage, or result from external pressure (rib notching). The two main causes of rib notching are coarctation of the aorta and neurofibromatosis Type I.
Ribs may fracture and the callus formed can sometimes mimic an intrapulmonary opacity.
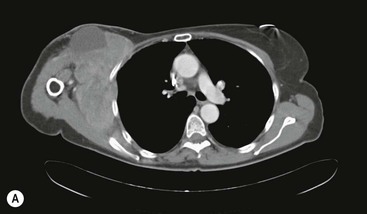
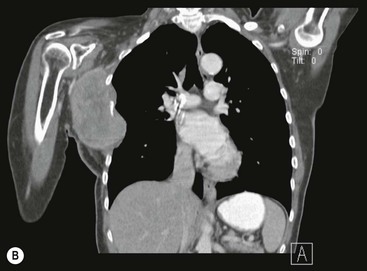
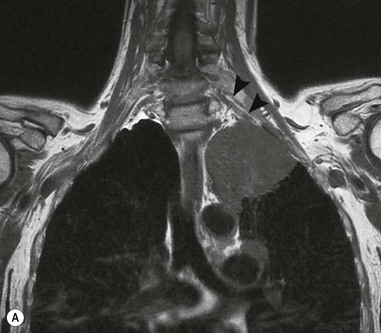
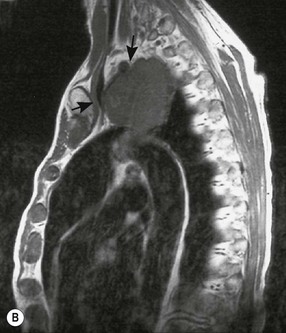
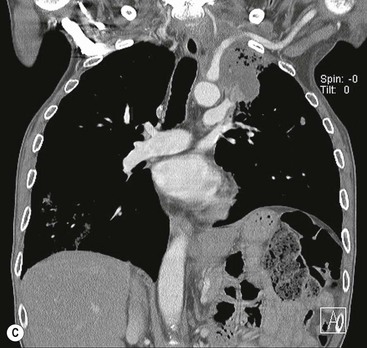
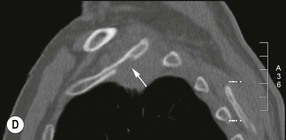
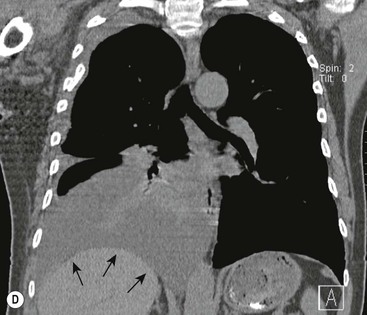
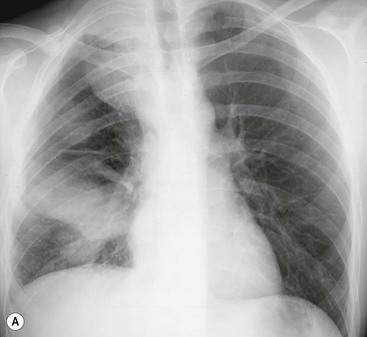
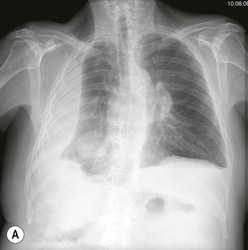
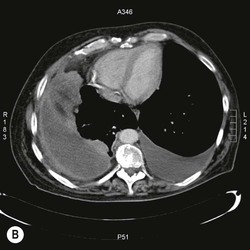
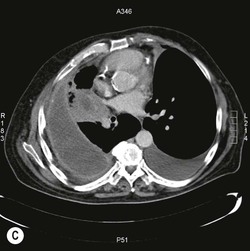
Destructive rib lesions occur most commonly in osteomyelitis or neoplastic disease. The former is uncommon and may be haematogenous (e.g. staphylococcal or tuberculous) or caused by direct spread from lung and pleural space (e.g. in actinomycosis). Bronchial carcinoma, including Pancoast’s tumour, commonly spreads from lung to rib. In this latter condition MRI can be performed to study the extent of the disease, especially the relationship between the tumour and the plexus brachialis in case of a Pancoast’s tumour3 (Fig. 10-3). Multidetector CT (MDCT) can also play an important role, especially because it can better evaluate the invasion in the bony cortex of the ribs4 (Fig. 10-3D). Also, 3D image reconstruction methods can be used in selected cases to clarify a complex relationship between the tumour invading the chest wall and vascular structures of the thoracic inlet.
Various primary and secondary tumours can affect ribs, causing localised lesions. Benign primary tumours are infrequent, and of these, the cartilaginous tumours (chondromas, osteochondromas) are the most common. They are predominantly anterior and may show characteristic cartilaginous calcification. Other lesions that broadly fall into this category include fibrous dysplasia, histiocytosis X, haemangioma and aneurysmal bone cyst1 (Fig. 10-4).
The most common malignant rib tumours are metastatic deposits and myeloma. Primary malignant tumours are rare, chondrosarcomas being the least uncommon. Other malignancies that occur occasionally include lymphoma, osteosarcoma and round-cell tumours.
Sternum
This is well displayed in a lateral chest radiograph but is conspicuous in the frontal projection, in which only the manubrial margins are sometimes visible, giving rise to confusing shadows that may mimic mediastinal widening.
Various sternal deformities are described, and the most important radiologically is the depressed sternum (funnel chest, pectus excavatum) in which there is approximation of the lower half of the sternum and the spine (Fig. 10-5). This may be an isolated abnormality or it may be associated with other disorders such as Marfan’s syndrome or congenital heart disease (particularly atrial septal defect (ASD)). The radiological signs on a postero-anterior (PA) chest radiograph consist of a shift of the heart to the left, straightening of the left heart border with prominence of the main pulmonary artery segment, loss of the descending aortic interface and an increased opacity in the right cardiophrenic angle, often accompanied by a loss of clarity of the right heart border which simulates right middle lobe disease. The diagnosis can be suspected on a PA radiograph from the steep inferior slope of the anterior ribs and undue clarity of the lower dorsal spine seen through the heart.
Pigeon chest (pectus carinatum) represents the reverse deformity and may be congenital or acquired.
Neoplasms of the sternum are usually malignant (myeloma, chondrosarcoma, lymphoma or metastatic carcinoma), the most common benign tumour being a chondroma. Non-neoplastic processes that may affect the sternum include osteomyelitis, histiocytosis X, Paget’s disease, fibrous dysplasia, osteitis fibrosa cystica and intersternocostoclavicular hyperostosis.
CT is the best investigation for imaging the sternum because it eliminates overlapping structures, detects bony destruction, allows imaging of adjacent soft tissues (the parasternal–internal mammary zone) and has good contrast resolution superior to that of conventional radiography or tomography.
Clavicles
The medial clavicular ends are important landmarks used together with the spine in assessing rotation on a radiograph.
The joints at both ends are synovial but only the acromio-clavicular joint can be assessed with confidence on a chest radiograph. It may be eroded in any synovitis, particularly rheumatoid arthritis, and is also commonly fuzzy and ill-defined in hyperparathyroidism and rickets. Neoplasms of the clavicle are usually malignant (myeloma or metastatic). Other primary tumours and tumour-like lesions include osteosarcoma, Ewing’s sarcoma, post-radiation sarcoma, aneurysmal bone cyst, histiocytosis X and intersternocostoclavicular hyperostosis. Either CT or MRI is required to provide a full evaluation of the medial clavicular ends.
Spine
Kyphoscoliosis makes assessment of the chest radiograph difficult and CT is often necessary to evaluate possible thoracic disease.
The Pleura
The chest radiograph is still the most important and widely used means of demonstrating and following the progress of pleural disease, though ultrasound, CT and MRI can play a significant role in a number of specific situations. Pleural disease is manifest by the accumulation of fluid or air in the pleural space, by pleural thickening (with or without calcification), or by the presence of a pleural mass.
Pleural Effusion
A number of different types of fluid may accumulate in the pleural space, the most common being transudate, exudate (thin or thick), blood and chyle. Occasionally effusions are highly specific, not falling into any of the above categories and containing, for example, bile, cerebrospinal fluid or iatrogenic fluids. All types of pleural effusion are radiographically identical, though historical, clinical and other radiological features may help limit the diagnostic possibilities. Sometimes, also CT and MRI can help to specify the diagnosis.
Bilateral pleural effusions tend to be transudates because they develop secondary to generalised changes that affect both pleural cavities equally—a rise in capillary pressure or a fall in blood proteins, etc. Some bilateral effusions are exudates, however, and this is seen with metastatic disease, lymphoma, pulmonary embolism, rheumatoid disease, systemic lupus erythematosus (SLE), post-cardiac injury syndrome, myxoedema and some ascites-related effusions. Right-sided effusions are typically associated with ascites, heart failure and liver abscess, and left effusions with pancreatitis, pericarditis, oesophageal rupture and aortic dissection. Massive effusions are most commonly due to malignant disease, particularly metastases (lung or breast), but may also occur in heart failure, cirrhosis, tuberculosis, empyema and trauma.
Imaging Pleural Effusion5
Chest Radiograph
Free Pleural Fluid.
A small amount of free fluid may be undetectable on an erect PA chest radiograph as it tends initially to collect under the lower lobes. Such small subpulmonary effusions can be demonstrated by ultrasound or CT.6
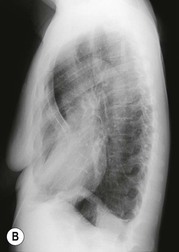
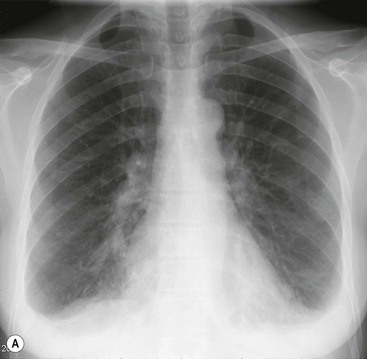
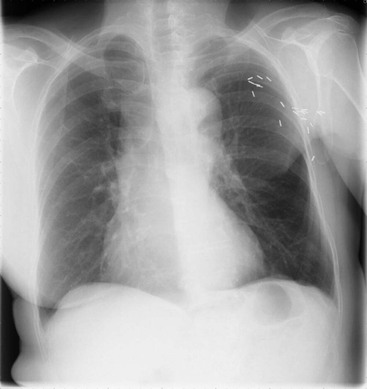
As the amount of effusion increases, the posterior and then the lateral costophrenic angles become blunted, by which time a 200- to 500-mL effusion is present. Following this the classical signs develop: homogeneous opacification of the lower chest with obliteration of the costophrenic angle and the hemidiaphragm. The superior margin of the opacity is concave to the lung and is higher laterally than medially. Above and medial to this meniscus there is a hazy increase in opacity owing to the presence of fluid posterior and anterior to the lungs (Fig. 10-6).
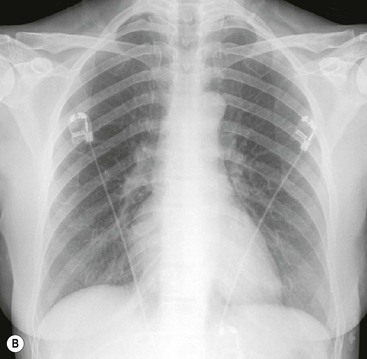
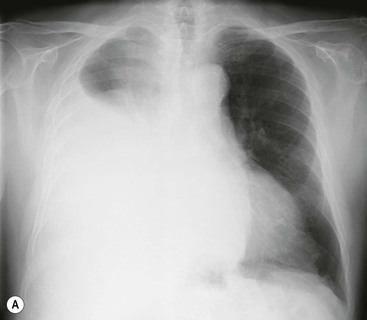
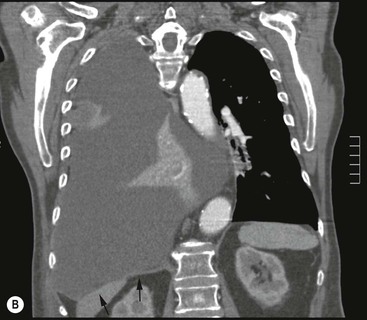
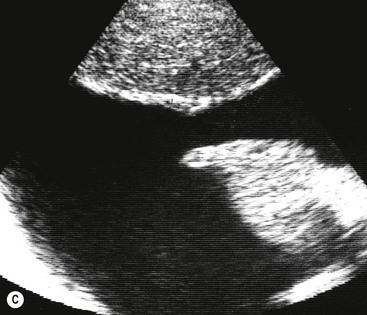
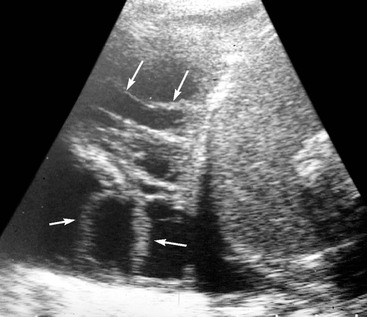
Massive effusions cause dense opacification of the hemithorax with contralateral mediastinal shift (Fig. 10-7). Absence of mediastinal shift with a large effusion raises the strong possibility of obstructive collapse of the ipsilateral lung or extensive pleural malignancy, such as may be seen with mesothelioma or metastatic carcinoma (Table 10-1). Large effusions sometimes cause diaphragmatic inversion, particularly on the left where the diaphragm lacks the support of the liver.7 Although pleural fluid collects initially under the lung, it is unusual for it to remain localised in this site once its volume exceeds 200–300 mL. This does happen occasionally, however, and may be suspected from an erect PA and lateral radiograph. On a PA radiograph this subpulmonary effusion7 presents as a ‘high hemidiaphragm’ with an unusual contour that peaks more laterally than usual, has a straight medial segment and falls away rapidly to the costophrenic angle laterally, which may or may not be blunted. Ultrasound or CT will confirm the diagnosis (see Fig. 10-8).
Loculated (Encysted, Encapsulated) Pleural Fluid.
Fluid can loculate between visceral pleural layers in fissures or between visceral and parietal layers, usually against the chest wall. It is unusual for this to happen without some additional radiographic clue to the presence of pleural disease (Fig. 10-9). Both ultrasound and CT can be used to distinguish loculated fluid from solid lesions.
Pleural Effusion in the Supine Patient.
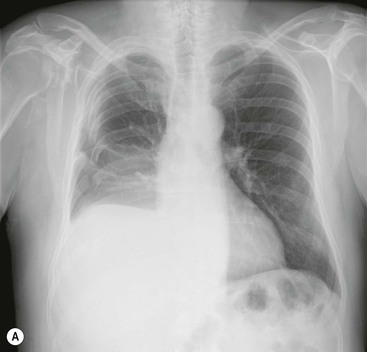
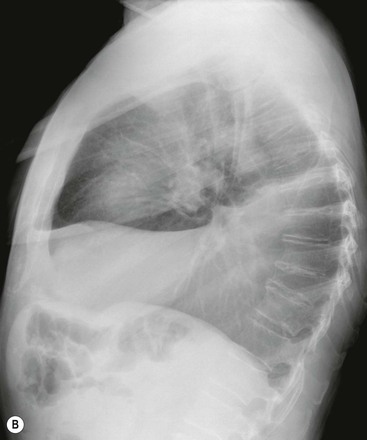
In the supine patient, pleural fluid layers out posteriorly and the meniscus effect, present from front to back, is not appreciated because of the projection. The main radiographic finding is a hazy opacity like a veil affecting the whole or the lower part of the hemithorax, with preserved vascular opacities in the overlying lung (see Fig. 10-6B). Additional signs include haziness of the diaphragmatic margin, blunting of the costophrenic angle, a pleural cap to the lung apex, thickening of the minor fissure and widening of the paraspinal interface.
Ultrasound 6,8
Pleural fluid, especially when it is a transudate, is commonly echo-free and marginated on its deep aspect by a highly echogenic line at the fluid–lung interface. Exudative and haemorrhagic effusions may be echogenic and are often accompanied by pleural thickening. The pattern of echoes may be homogeneous, complex or septated. Features that help distinguish a fluid from a solid echogenic lesion include changes in shape with breathing, the presence of septa and fibrous strands and movement of components induced by breathing (Fig. 10-10). Occasionally, in the absence of such features, some echogenic fluid pleural effusions are indistinguishable from solid ones.6
Ultrasound has a number of important roles in the evaluation and management of pleural fluid. It can be used to distinguish between pleural fluid, solid pleural (or extrapleural) lesions and peripheral lung lesions. In peripheral lung lesions, the presence of fluid bronchograms and vessels on Doppler examination will positively identify consolidation. In addition, pleural lesions characteristically make an obtuse angle with the chest wall, whereas with intrapulmonary lesions the angle is often acute. This ability of ultrasound to distinguish pulmonary lesions (collapse, consolidation, abscess) from pleural effusion is particularly useful when it comes to the evaluation of the opaque hemithorax. Ultrasound can also be used to identify small amounts of pleural fluid, or pleural fluid in unusual locations, as with a subpulmonary effusion (see Fig. 10-8C). Ultrasound is widely used to localise pleural fluid for aspiration and identify any solid components to allow guided biopsy. Furthermore, ultrasound may identify the cause of an effusion when it lies inside or even outside the chest (subphrenic abscess, metastasis, etc.).
Computed Tomography 6,9
CT is very sensitive in detecting pleural fluid and can distinguish between free and loculated fluid, identifying the extent and location of the latter. Accurate localisation of such loculated effusions is useful before drainage. CT distinguishes between parenchymal lung disease and pleural disease, a distinction often facilitated by administration of intravenous contrast medium. CT can characterise the morphology of pleural thickening that often accompanies a pleural effusion, distinguishing between malignant (nodular, with focal masses) and benign thickening, which is typically uniform. CT can also identify any underlying lung disease that might have provoked an effusion and it facilitates percutaneous aspiration and biopsy (Fig. 10-11).
A pleural effusion appears on CT as a dependent sickle-shaped opacity with a CT number lower than that of any adjacent pleural thickening or mass. CT numbers do not allow a distinction between transudate and exudate. However, parietal pleural thickening at contrast-enhanced CT almost always indicates the presence of pleural exudates. The higher density of clotted blood in a haemothorax is sometimes apparent. The fat-containing chylothorax does not have a CT number lower than normal, because of its protein content. Loculated effusions have a lenticular configuration with smooth margins and they displace the adjacent parenchyma.
Magnetic Resonance Imaging6
MRI has a limited role in the evaluation of pleural effusion. Pleural fluid has a low signal on T1-weighted sequences and a high signal on T2-weighted images, with a tendency for exudates to give a higher signal than transudates on T2-weighted sequences. In addition, complex exudates have greater signal intensity than simple exudates. It may also be possible to differentiate transudates from exudates using triple echo-pulse sequence, and benign from malignant changes using high-resolution MRI.10 Chylous effusion can cause high signal intensity on T1-weighted images similar to subcutaneous fat. In the subacute and chronic stage, haematomas show bright signal intensity on T1-weighted images, surrounded by a dark rim caused by haemosiderin.
Some Specific Pleural Effusions
Exudates and Transudates
Pleural effusion is common in heart failure and tends to be more frequent and larger on the right.
All types of pericardial disease may be associated with pleural effusion, which is predominantly left-sided. Pleural effusion is a characteristic finding in the post-cardiac injury syndrome, seen in about 80% of patients. It may be bilateral or unilateral and is commonly accompanied by consolidation and pericardial effusion. Pulmonary embolism is commonly associated with pleural effusion, which is seen in 25–50% of cases.
A number of drugs have been described as causing pleural effusions. The most common agents are cytotoxics (methotrexate, procarbazine, mitomycin, busulfan, bleomycin and interleukin-2), nitrofurantoin, antimigraine drugs (ergotamine, methysergide), amiodarone, propylthiouracil, bromocriptine and gonadotrophins. With a number of these agents pleural thickening is more common than a pleural effusion.
Pleural effusion is also a recognised complication of hepatic cirrhosis. The principal mechanism of its production is the transdiaphragmatic passage of ascites, though other factors such as hypoalbuminaemia may contribute in a small number of cases.
Both acute and chronic pancreatitis are associated with pleural effusions which have high amylase levels. In acute pancreatitis, exudative and often blood-stained effusions form in 15% of patients, particularly on the left side where the diaphragm is closely related to the pancreatic tail. Associated elevation of the hemidiaphragm and basal lung consolidation are common. In chronic pancreatitis, effusions tend to be large and recurrent and patients present with dyspnoea, unlike effusions in acute pancreatitis in which abdominal symptoms predominate. The pathogenesis of pleural effusion in chronic pancreatitis is fistula formation following ductal rupture.
Pleural effusion is common with subphrenic abscess and occurs in about 80% of patients. The effusion is often accompanied by basal lung collapse and consolidation, an elevated hemidiaphragm and a subdiaphragmatic air–fluid level.
Pleural effusion may occur in a number of renal conditions. Exudative effusions may be seen in uraemia and are often accompanied by pericarditis. Effusions can be large or small and are often unilateral, behaving in a rather indolent fashion. In common with other hypoproteinaemic states, bilateral effusions develop in about 20% of patients with nephrotic syndrome. Peritoneal dialysis can produce pleural effusions by the direct transdiaphragmatic passage of fluid, as occurs with cirrhotic ascites. In common with other ascites-related effusions they are predominantly right-sided, but these effusions have a diagnostically high level of glucose.
Patients with acquired immune deficiency syndrome are at risk for a variety of pleural infections and neoplasms that can be associated with pleural effusion. These effusions are most frequently caused by pneumonic infections but can also be the result of non-Hodgkin’s lymphoma. Empyema is a suppurative exudate usually parapneumonic. Less commonly it is caused by transdiaphragmatic extension of a liver abscess or by bronchopleural fistula (Fig. 10-12).