Invasive lobular carcinoma (ILC) of the breast is, due to its diffuse infiltrative growth pattern, a diagnostic challenge. Even in retrospect, only up to 80% are visible at mammography. Moreover, both mammography and ultrasound tend to structurally underestimate the size of ILC. Breast magnetic resonance (MR) imaging is usually performed after initial cancer detection. In this setting, the sensitivity is approximately 96%. However, multiple cases have been reported in which ILC has been initially detected with MR imaging, thus implying a potential advantage of MR imaging over mammography in screening. The size of an ILC as reported on MR imaging correlates well with size at pathology ( r = 0.89). Additional tumor foci are detected by MR imaging in approximately one-third of patients, and these foci are subsequently pathologically confirmed in 88%. Hence, preoperative MR imaging of ILC changes management in 28% of patients, often appropriately. Nevertheless, it is still essential to obtain histology prior to large changes in the therapeutic regime based on MR imaging findings, either by second-look ultrasound or by MR imaging–guided biopsy. Using this approach, it has been shown that preoperative MR imaging reduces the rate of reexcisions after breast-conserving surgery from 27% to 9%, without increasing the rate of mastectomies and without extending total therapy time. Finally, the early detection of contralateral carcinomas only visible at MR imaging in approximately 7% of patients with ILC implies that preoperative MR imaging in these patients improves survival, although the magnitude of this effect is unknown.
Invasive lobular carcinoma (ILC) is the second most common form of breast cancer, reported in 5% to 20% of patients. The relative frequency of ILC has been increasing in the last decades, probably related to the increased use of complete hormone replacement therapy in perimenopausal women. The reduced use of this therapy in recent years may already have resulted in a small decrease of the incidence of ILC. ILC derives its name from the old assumption that the tumor arises from the lobules, whereas the more common form of breast cancer, invasive ductal carcinoma (IDC), arises from the milk ducts. Because most breast cancers, including IDC and ILC, have been shown to arise from the terminal ductal lobular units, these common breast cancers are somewhat awkwardly named.
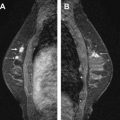
The main difference between IDC and ILC is their growth pattern, with ILC tending to grow more diffusely. The “classic type” lobular carcinoma consists of relatively small, uniform cells that grow in a loosely cohesive fashion, forming lines of cells infiltrating the healthy tissue—so-called Indian files ( Fig. 1 ). Formation of webs around healthy ducts, referred to as targetoid growth, is often reported. Furthermore, skip lesions, that is, areas of tumor separated from the index lesion by normal breast tissue, are more common than in IDC. Moreover, synchronous and metachronous contralateral carcinomas are more often observed in ILC.

The genetic basis for these differences is probably due to a mutation in the E-cadherin gene (CDH1). E-cadherin is strongly related to cell-cell cohesion, and affects morphology and motility of cells. Hence a lack of E-cadherin expression may be the cause for the disjointed growth of ILC. Apart from the lack of E-cadherin expression, classic ILC biologically resembles low-grade IDC. Similarly, the more aggressive subtype pleomorphic ILC resembles high-grade IDC.
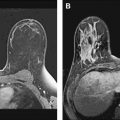
There are only a few other documented differences between IDC and ILC. ILC are generally larger at detection than IDC, and are more often estrogen and progesterone receptor positive. Furthermore, ILC metastasizes to locations that are extremely rare for IDC, such as the gastrointestinal tract, the retroperitoneum, the gynecologic organs, and the leptomeninges. However, the most common metastatic sites for ILC are the lungs, the liver, and the bones ( Figs. 2–5 ).




Outcomes are not very different, with a 5 year disease-free survival of 85.7% and 83.5% for ILC and IDC, respectively. Some studies suggest even a slightly better outcome for ILC than IDC, regardless of the often larger size of ILC at diagnosis. At present, there are no differences in treatment based on the histopathologic differentiation between IDC and ILC.
Despite the relative small differences between IDC and ILC, ILC presents a major diagnostic challenge. The tumors are, due to their diffuse growth pattern, more difficult to detect than IDC. The infiltrative growth pattern is the most likely explanation for why ILC tends to be larger than IDC. Moreover, the diffuse growth pattern of ILC makes mammography and ultrasound unreliable at staging, thus causing high rates of tumor reexcision and leading to a common preference by both patients and surgeons to perform mastectomy. Fortunately, studies have shown that mastectomy rates for ILC are decreasing.
Because breast MR imaging has been shown to be better at tumor staging, its use may be especially valuable in the preoperative staging of the subgroup of patients with ILC.
Conventional imaging methods in ILC
In the first evaluation of interval cancers after the initiation of breast cancer screening with mammography in the Netherlands, it became clear that ILC was a common pathologic diagnosis in the missed carcinoma group. This finding was attributed to the diffuse infiltrative pattern of the tumors and the poor desmoplastic reaction of the surrounding tissue. In a later, larger study, about one-third of the interval carcinomas were of lobular origin. In a recent evaluation that differentiated between “true” interval carcinomas (fast-growing tumors not present at the time of screening) and false-negative screening mammography, 47% of the latter category were tumors with lobular features. This finding may be due to ILC more often being better visualized on craniocaudal (CC) mammographic images than mediolateral oblique (MLO) images, whereas the former are not routinely performed in all screening programs. However, even in retrospect 10% to 20% of ILC is not visible at mammography.
The hallmark of malignancy on mammography, a spiculated mass, is reported in 28% to 63% of ILC cases ( Figs. 6 and 7 ). Mammographic findings in the remainder of ILC cases are often subtle. There is no association of ILC with microcalcifications, and the tumors are often isodense to fibroglandular tissue.


Common descriptors include ill-defined mass (7%–33%), architectural distortion (10%–24%), and asymmetry (4%–14%). The wide ranges reported probably reflect interreader variability of the descriptive terminology.
Tumor size estimation with mammography is difficult. Reported correlation coefficients range widely from 0.2 to 0.8. Small tumors in fatty breasts are quite accurately assessed, but accuracy decreases rapidly with increasing tumor size and increasing density, resulting in structural underestimation of larger tumors. The vague borders commonly seen in ILC make assessment of these tumors particularly difficult, which results in measurements with a stronger negative deviation from pathologic tumor size when compared with those seen in IDC. Consequently, it has been shown that mammography understages more than one-third of ILC.
Sonography is hardly ever used as a screening modality, hence studies that report on sensitivity of ultrasound in ILC report on lesions that have already been detected by other means (either physical examination or mammography).
Nevertheless, most ILC are visible at sonography, and reported sensitivities range from 78% to 98%. In a recent meta-analysis comparing the sensitivity of ultrasound directly with MR imaging, the sensitivity of ultrasound was 83%, with a 95% confidence interval ranging from 71% to 91%.
Although one initial study reported difficulties with the detection of ILC smaller than 1 cm (only 1 out of 4), later studies using more sophisticated equipment reported sensitivities in the normal range. Of note, ultrasound sensitivity is also high in lesions that are hardly visible or occult at mammography. Hence ultrasound has complementary value for the detection of ILC in the symptomatic patient. Approximately 60% of ILC lesions exhibit the typical features of malignancy at ultrasound, and present as a hypoechoic heterogeneous mass with ill-defined margins and posterior acoustic shadowing ( Fig. 8 ). Internal hyperechoic patterns are more commonly seen in ILC than in IDC (see Fig. 8 D), and some ILC tumors present as areas of focal shadowing without a discrete mass. This latter ultrasound appearance may suggest the classic type of ILC histology.

Regarding tumor size estimation of ILC, ultrasound performs equally well as mammography, although the spread of reported correlation coefficients is slightly lower, ranging from 0.5 to 0.8. Similar to mammography, the quality of the tumor size assessment decreases with increasing size of tumor; this holds particularly true for ILC tumors larger than 3 cm, which cannot be accurately assessed with ultrasound.
Features of ILC on breast MR imaging
The retrospective sensitivity of breast MR imaging for ILC is high. In a meta-analysis evaluating studies published until April 2006 describing in total 209 patients, the sensitivity was 93.3%, with a 95% confidence interval (CI) ranging from 88% to 96%. Leaving out the results of one very early study that scanned their patients with a far from optimal scan protocol, the sensitivity was even higher, at 96% (95% CI 92%–98%). Since the publication of this meta-analysis, to the author’s knowledge, 3 new studies have appeared in the literature that allowed evaluation of sensitivity. The 2 largest studies both reported a retrospective sensitivity of 100% (in 57 and 69 patients, respectively). The third study, which was actually aimed at the evaluation of breast-specific gamma imaging for the detection of ILC, reported 2 false negatives in a series of only 12 patients, resulting in a sensitivity of 83% ; this can only be explained by bad luck, as it is far less than the earlier reported confidence intervals.
Almost all available studies are retrospective in design. Only two studies completely report prospective data, and one study is partly prospective. Francis and colleagues reported a sensitivity of 95% in 22 ILC, Berg and colleagues reported a sensitivity of 97% in 29 ILC, and Caramella and colleagues reported a sensitivity of 100% in 35 ILC. Hence, prospective data are well in line with the results from the retrospective studies.
Nevertheless, all studies evaluated patients that were known to have a carcinoma. Although various investigators report ILC that were incidentally detected in MR imaging examinations performed for other indications, few data are available that report the sensitivity of breast MR imaging for ILC in a screening situation. In general, the sensitivity of breast MR imaging for breast cancer in screening is lower than in preoperative staging, though much better than mammography; reported sensitivities range from 77% to 100%. In the large screening studies too few ILC were detected to produce conclusive results; however, Kriege and colleagues reported a sensitivity of 100% for MR imaging in the detection of 4 ILC, compared with 25% for mammography, suggesting an additional value over mammography of screening for ILC with MR imaging.
For optimal detection of ILC in a screening setting, it is essential to know the appearance of ILC on breast MR imaging. It is commonly stated that ILC appears more often as nonmass-like enhancement and in general enhances less than IDC. At the same time good scientific evidence for these statements is lacking, partly because the interpretation of breast MR imaging, even using the rigorous approach of the BI-RADS lexicon, is subject to considerable interreader variability. As a direct consequence, the principal distinction between mass-like and nonmass-like lesions is a very difficult one to make. Different studies report the incidence of nonmass-like enhancement to be between 5% and 69%. Pooling of these data is not possible because of the large heterogeneity in the studies.
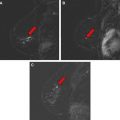
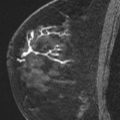
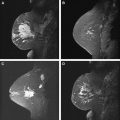
In the large group of mass-like lesions, about 85% are described as irregular and spiculated. Therefore, an irregular spiculated mass is in fact the most common appearance of ILC on MR imaging ( Fig. 9 ). However, round masses with sharp margins have also been described, which subsequently turned out to be ILC. Nonmass-like enhancement can be either ductal, segmental, regional, or diffuse ( Fig. 10 ).

Evaluation of the enhancement pattern has less often been described. Sittek and colleagues and Trecate and colleagues both noted that peak enhancement was reached relatively late, and washout in the late phase of enhancement was uncommon. Caramella and colleagues noted continuous enhancement in the late phase of enhancement (commonly referred to as a type 1 curve) in 37% of ILC. Two studies that evaluated quantitative enhancement parameters also noted that these values appeared much lower for ILC than in other studies evaluating the same parameters for IDC.
In the absence of studies that directly compare morphologic and kinetic descriptors between IDC and ILC, the magnitude of the differences between the appearances of ILC and IDC cannot be adequately assessed. Such direct comparison studies have been performed and reported on, but are so far unpublished.
Newstead and colleagues presented a comparison of 22 ILC with 257 IDC and 83 ductal carcinoma in situ (DCIS) lesions at the 2005 meeting of the Radiological Society of North America. These investigators reported that 55% of ILC presented as a mass, compared with 76% of IDC and only 16% of DCIS lesions. Time to peak enhancement was twice as long for ILC as for IDC (270 ± 112 seconds vs 131 ± 90 seconds) and enhancement after 68 seconds was consequently lower in ILC than in IDC.
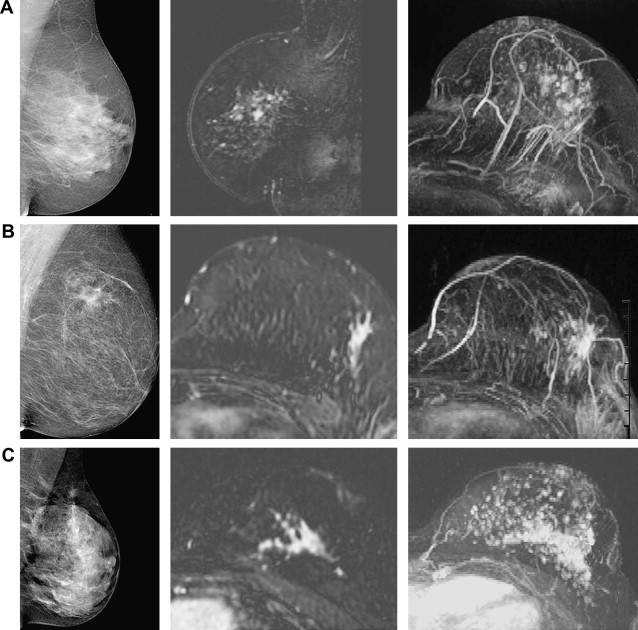
Mann and colleagues reported at the 2008 meeting of the International Society for Magnetic Resonance in Medicine a comparison of 33 ILC with 103 IDC, in which 75% of ILC presented as a mass compared with 84% of IDC. Interreader variability was moderate (κ = 0.41), comparable to literature values, and similar for ILC and IDC. Peak enhancement was not different between ILC and IDC (360% vs 382%), but at visual assessment washout was less common in ILC (48% vs 84%). Using a computer-aided diagnosis (CAD) application, this difference was blotted out; washout was detected in 88% of ILC and 94% of IDC, suggesting that CAD applications may be especially helpful in the assessment of ILC ( Fig. 11 ). This finding is explained by the observation that the fraction of the lesion that shows washout is generally smaller in ILC (<10% of the dominant focus in 64% of ILC vs 30% of IDC). The results of pharmacokinetic analysis also showed that ILC in general enhance slower than IDC, but not less.
Lastly, at the 2009 European Congress of Radiology, Dietzel and colleagues reported on a comparison of 108 ILC with 347 IDC. In their series ILC were more often irregular lesions than IDC (62% vs 55%), though this did not reach statistical significance. An essential finding was, however, that internal necrosis (and hence ring enhancement) was less common in ILC than in IDC (3% vs 15%) and that perifocal edema was less often observed (30% vs 45%). Moreover, they also noted that washout was less frequent in ILC than in IDC (57% vs 73%). Both tumor types were nearly always iso- to hypointense compared with glandular breast tissue on T2-weighted imaging.
In summary, almost all ILC are retrospectively visible. Most ILC still present as an irregular spiculated mass, but the frequency of nonmass-like enhancement (between 20% and 40% approximately) is slightly higher than in IDC. Ring enhancement and surrounding edema are less frequently observed. Contrast enhancement is slower than in IDC, but not necessarily less, which results in a higher proportion of lesions that do not show a typical washout curve. CAD applications may help to adequately assess the most malignant curve shape; however, the morphologic appearance is usually that of a suspicious lesion and should not be misinterpreted in the absence of a washout curve.
Features of ILC on breast MR imaging
The retrospective sensitivity of breast MR imaging for ILC is high. In a meta-analysis evaluating studies published until April 2006 describing in total 209 patients, the sensitivity was 93.3%, with a 95% confidence interval (CI) ranging from 88% to 96%. Leaving out the results of one very early study that scanned their patients with a far from optimal scan protocol, the sensitivity was even higher, at 96% (95% CI 92%–98%). Since the publication of this meta-analysis, to the author’s knowledge, 3 new studies have appeared in the literature that allowed evaluation of sensitivity. The 2 largest studies both reported a retrospective sensitivity of 100% (in 57 and 69 patients, respectively). The third study, which was actually aimed at the evaluation of breast-specific gamma imaging for the detection of ILC, reported 2 false negatives in a series of only 12 patients, resulting in a sensitivity of 83% ; this can only be explained by bad luck, as it is far less than the earlier reported confidence intervals.
Almost all available studies are retrospective in design. Only two studies completely report prospective data, and one study is partly prospective. Francis and colleagues reported a sensitivity of 95% in 22 ILC, Berg and colleagues reported a sensitivity of 97% in 29 ILC, and Caramella and colleagues reported a sensitivity of 100% in 35 ILC. Hence, prospective data are well in line with the results from the retrospective studies.
Nevertheless, all studies evaluated patients that were known to have a carcinoma. Although various investigators report ILC that were incidentally detected in MR imaging examinations performed for other indications, few data are available that report the sensitivity of breast MR imaging for ILC in a screening situation. In general, the sensitivity of breast MR imaging for breast cancer in screening is lower than in preoperative staging, though much better than mammography; reported sensitivities range from 77% to 100%. In the large screening studies too few ILC were detected to produce conclusive results; however, Kriege and colleagues reported a sensitivity of 100% for MR imaging in the detection of 4 ILC, compared with 25% for mammography, suggesting an additional value over mammography of screening for ILC with MR imaging.
For optimal detection of ILC in a screening setting, it is essential to know the appearance of ILC on breast MR imaging. It is commonly stated that ILC appears more often as nonmass-like enhancement and in general enhances less than IDC. At the same time good scientific evidence for these statements is lacking, partly because the interpretation of breast MR imaging, even using the rigorous approach of the BI-RADS lexicon, is subject to considerable interreader variability. As a direct consequence, the principal distinction between mass-like and nonmass-like lesions is a very difficult one to make. Different studies report the incidence of nonmass-like enhancement to be between 5% and 69%. Pooling of these data is not possible because of the large heterogeneity in the studies.
In the large group of mass-like lesions, about 85% are described as irregular and spiculated. Therefore, an irregular spiculated mass is in fact the most common appearance of ILC on MR imaging ( Fig. 9 ). However, round masses with sharp margins have also been described, which subsequently turned out to be ILC. Nonmass-like enhancement can be either ductal, segmental, regional, or diffuse ( Fig. 10 ).