Chapter 1 The Elements of Cardiac Imaging
Cardiovascular imaging is different from that for all other organs because the dimension of time has to be included in the subsecond acquisition and analysis of images. The chest film remains the entry-level examination for most cardiac problems. Although daunting economic and scheduling constraints remain, the cross-sectional methods—echocardiography, computed tomography (CT), and magnetic resonance imaging (MRI)—are becoming the primary imaging choices to diagnose cardiac diseases because the millisecond temporal resolution and the millimeter spacial resolution can follow the beating heart and the moving blood.
THE CHEST FILM
The chest film is often the first imaging procedure performed when heart disease is suspected, and more commonly, it is used to assess and follow the severity of cardiac disease. Because the chest film forms images by projection, this technique detects only those cardiopulmonary abnormalities that change the shape of the heart, mediastinum, and lungs and those that alter the structure of the pulmonary vasculature. Clinically silent heart disease may also be detected on a chest film taken for other reasons. Extracardial structures, particularly in the abdomen and the thoracic cage, may produce additional clues indicating heart disease. Calcification in the aortic valve, for example, identifies the abnormal structure and directs the differential diagnosis toward a particular pathologic lesion (Box 1-1).
CARDIAC SHAPE AND SIZE
Age and Its Visible Effects
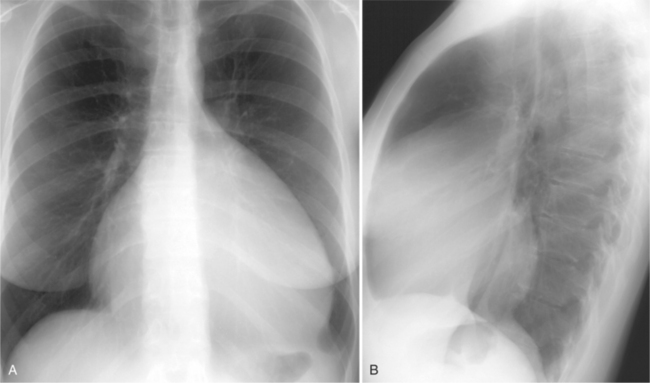
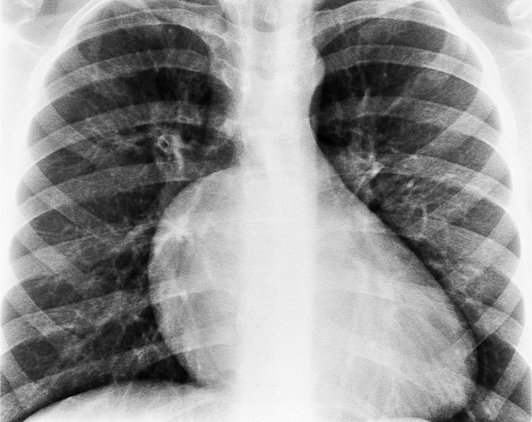
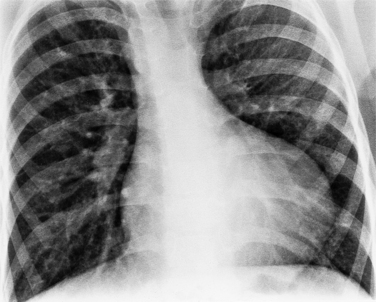
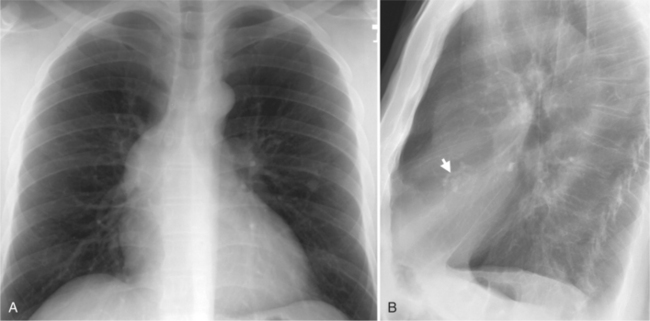
In the child and adolescent, the bronchopulmonary markings become more distinguishable, and the thymic shadow regresses and becomes inapparent so the aortic arch and pulmonary trunk can be seen. A convex pulmonary trunk in girls in their late teens may suggest pulmonary artery enlargement, but in the absence of a heart murmur this is usually a normal variant (Fig. 1-1). However, an electrocardiogram (ECG) may be necessary to exclude entities such as pulmonary stenosis and left-to-right shunts. The “double density” of the pulmonary veins may mimic an enlarged left atrium (Fig. 1-2), but a large left atrium has a rounder curve and extends medially above the diaphragm.
Evaluation of Heart Size
Cardiothoracic Ratio
The determination of heart size, both subjectively and quantitatively, has been assessed from the chest film for more than 70 years. Then Danzer described the cardiothoracic ratio, which is still one of the most common measurements of overall heart size. This ratio was constructed to measure left ventricular dilatation. Because it measures the transverse heart diameter, the cardiothoracic ratio is usually normal when either the left atrium or the right ventricle is moderately enlarged because neither of these two chambers is reflected in the transverse dimension. The left atrium and right ventricle become border-forming when they are severely enlarged. Rose and colleagues noted that for the cardiothoracic ratio to reliably detect enlargement of the left ventricle (Table 1-1), changes in left ventricular volume up to 66% in excess of normal are needed.
TABLE 1-1 Cardiothoracic ratio
| Patient Characteristics | Normal Ratio |
| Newborn | <0.6 |
| >1 month old | <0.5 |
| Sensitivity = 0.45 (Many patients with left ventricular dilatation are not detected.) | |
| Specificity = 0.85 (When ratio exceeds the normal value, heart is clearly large.) | |
| Accuracy = 0.59 | |
Modified with permission from Rose CP, Stolberg HO: The limited utility of the plain chest film in the assessment of left ventricular structure and function, Invest Radiol 17:139-144, 1982.
Measurements of the heart and mediastinum are dramatically affected by the height of the diaphragm and the intrathoracic pressure and less so by the body position and status of the intravascular volume (Table 1-2).
TABLE 1-2 Typical variations of heart and mediastinum measurements on the chest film
| Circumstance | Variation |
|---|---|
| In expiration | Transverse diameter of heart and mediastinum widens Indistinct appearance of pulmonary hilum can be identical to that seen with pulmonary edema |
| In recumbent position | Heart is broader Lung volumes are lower Upper lobe arteries and veins appear more distended |
| On posteroanterior film | Change in heart width between systole and diastole is typically less than 1 cm |
| On right anterior oblique film | Heart size does not change between systole and diastole Left ventricular apex appears akinetic |
| On left anterior oblique film | Posterolateral wall motion is typically more than 1 cm |
Chamber Enlargement
Each chamber basically enlarges directly outward from its normal position. Except for the right ventricle, isolated chamber enlargement does not affect the position of the heart in the mediastinum or the identification of other chamber enlargement. When the right ventricle enlarges, it contacts the sternum and rotates the heart posteriorly and in a clockwise direction as viewed from below. Frequently in right ventricular enlargement, the normal left ventricle may falsely appear enlarged on both the frontal and lateral films because the entire heart is displaced posteriorly. If the right ventricle is dilated, the diagnosis of left ventricular enlargement may not be possible in the chest film (Dinsmore principle). Therefore, you should assess the size of the right ventricle on the lateral film before judging the left ventricle (Figure 1-3, Box 1-2).
Box 1-2 Right atrial enlargement on chest film
Right Atrium
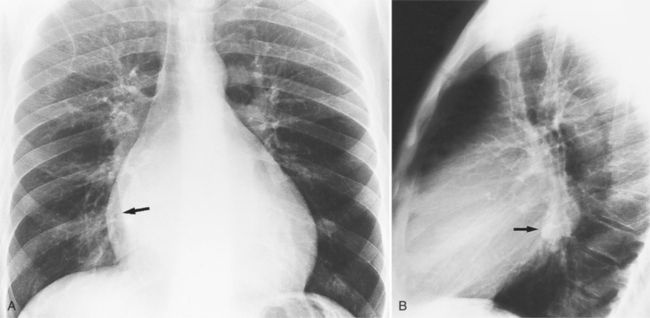
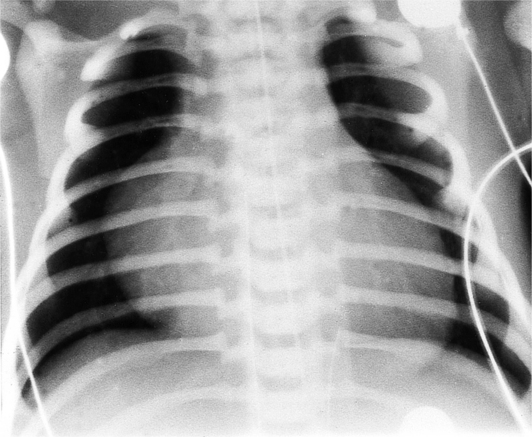
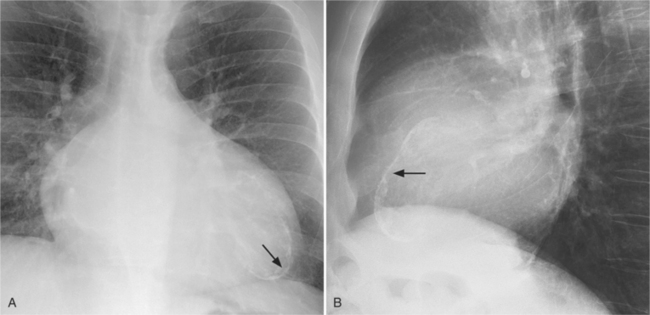
In the frontal view, the right atrium is visible because of its border with the right middle lobe (see Box 1-2). Neither subtle nor moderate enlargement can be recognized accurately because there is moderate variability of its shape in normal subjects, and in expiration the right atrium becomes rounder and moves to the right (Figures 1-4, 1-5).
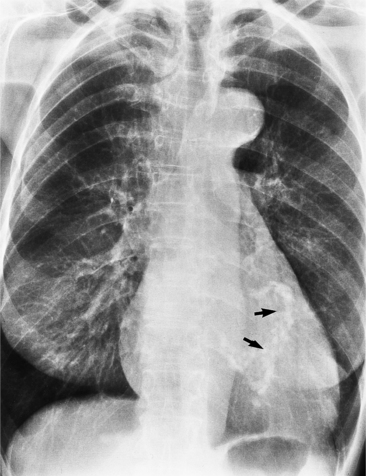
The right atrium and the other three chambers enlarge because of increased pressure, increased blood volume, or a wall abnormality. Common causes of right atrial enlargement are tricuspid stenosis and regurgitation, atrial septal defect, atrial fibrillation, and dilated cardiomyopathy. Ebstein anomaly may have all of these features. In pulmonary atresia, the right atrium dilates in direct proportion to the amount of tricuspid regurgitation (Fig. 1-6).
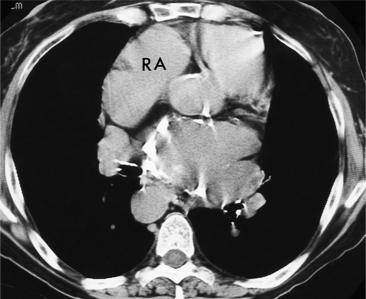
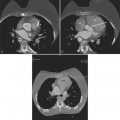
All the signs of right heart enlargement that are implied on the chest film are directly visible on the CT scan. The right atrium and ventricle touch the anterior chest wall and rotate the heart posteriorly. The right coronary artery adjacent to the right atrial appendage lies to the left of the sternum (Fig. 1-7).
Right Ventricle
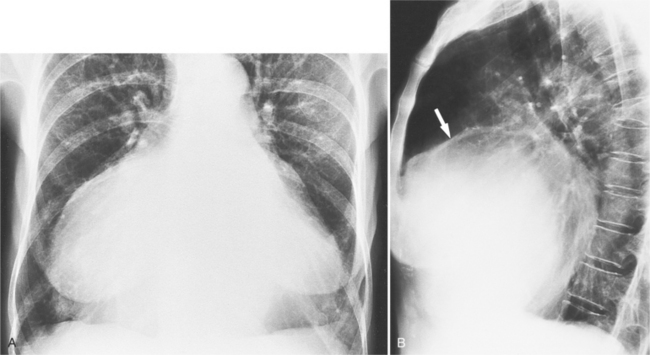
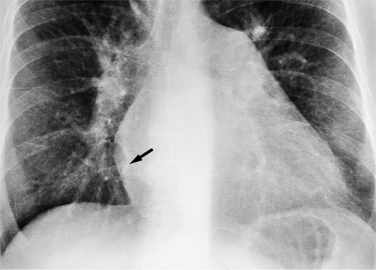
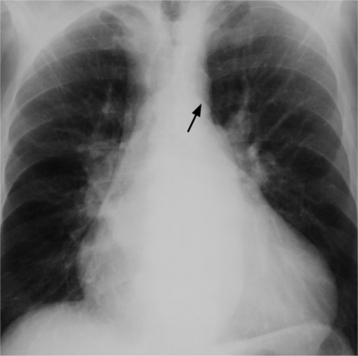
On the lateral view, the normal right ventricle does not touch more than one fourth of the lower portion of the sternum as measured by the distance from the sterno diaphragmatic angle to the point at which the trachea meets the sternum. One sign of right ventricular enlargement is the filling in of more than one third of the retrosternal space. On the frontal view, the normal right ventricle is not visible, and only extreme dilatation causes recognizable signs because the heart rotates clockwise as it dilates and pushes against the sternum. In this instance, the usual contour of the left atrial appendage is rotated posteriorly and is no longer part of the left side of the mediastinum. You can recognize this sign by an unusually long convex curvature extending inferiorly from the main pulmonary artery (Fig. 1-8). In extreme instances the entire left heart border may be the right ventricle (Box 1-3).
Box 1-3 Right ventricular enlargement on chest film
Frontal view (the right ventricle is usually not visible on the frontal view)
In tetralogy of Fallot when the fat pad is absent in the left cardiophrenic angle, the heart may have an uplifted cardiac apex (Fig. 1-9), which has been called the “boot-shaped heart” or the coeur en sabot. The right ventricle is not enlarged but may have hypertrophy.
Left Atrium
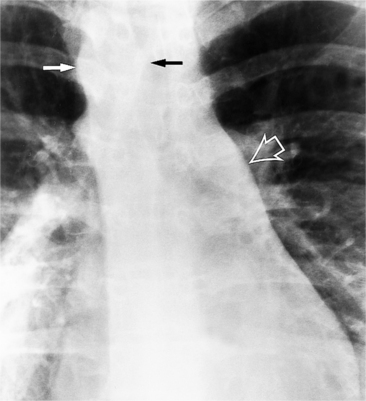
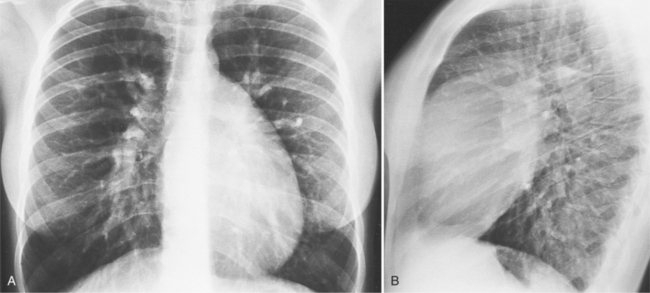
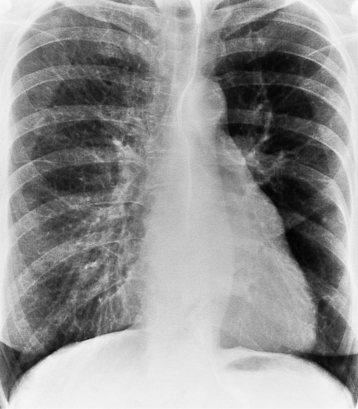
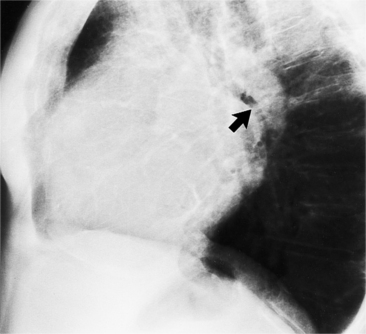
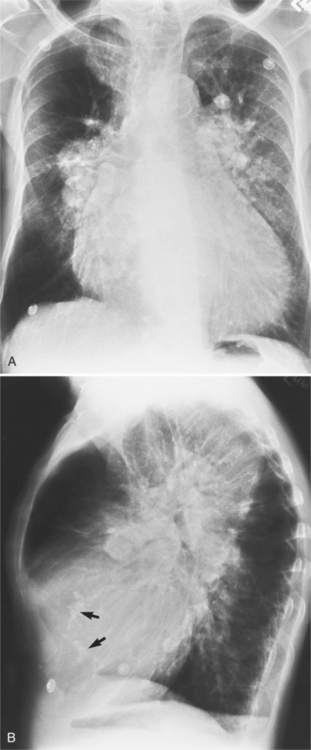
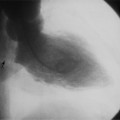
There are many clues to left atrial enlargement on the frontal and lateral chest film. One of the earliest signs of slight enlargement is the appearance of the double density, which is the right side of the left atrium as it pushes into the adjacent lung. Because a prominent pulmonary vein or varix may also cause a vertical double density, the double density should begin to curve inferiorly (Fig. 1-10). In extreme cases, the left atrium may enlarge to the right side and touch the right thoracic wall (Fig. 1-11). The etiology of this “giant left atrium” is rheumatic heart disease, mainly from mitral regurgitation.
A convex left atrial appendage on the frontal view is abnormal and usually reflects prior rheumatic heart disease. In pure mitral regurgitation, the body of the left atrium, not the appendage, enlarges.
The indirect signs visible only when the left atrium is dilated at least moderately are highlighted in Box 1-4 and Figures 1-12 and 1-13.
Box 1-4 Left atrial enlargement on chest film
Common acquired causes of left atrial enlargement are mitral stenosis or regurgitation, left ventricular failure, and left atrial myxoma. Congenital causes include ventricular septal defects, patent ductus arteriosus, and the hypoplastic left heart complex. When atrial fibrillation occurs, the left atrial volume may increase by 20%.
Left Ventricle
Left ventricular enlargement exists if the left heart border is displaced leftward, inferiorly, or posteriorly. Inferior displacement may invert the diaphragm and cause this border to appear in the gastric air bubble. The chest film cannot reliably distinguish between left ventricular dilatation and hypertrophy. With hypertrophy, the apex has a pronounced rounding and a decrease in its radius of curvature. The elderly normal heart also has this shape. When massive hypertrophy is present, the left ventricular shape is large and appears similar to one that is only dilated (Box 1-5).
Common causes of left ventricular enlargement can be grouped into three categories: pressure overload (hypertension, aortic stenosis; Fig. 1-14); volume overload (aortic or mitral regurgitation, ventricular septal defects; Fig. 1-15); and wall abnormalities (left ventricular aneurysm, hypertrophic cardiomyopathy; Fig. 1-16).
CARDIAC AND PERICARDIAL CALCIFICATIONS
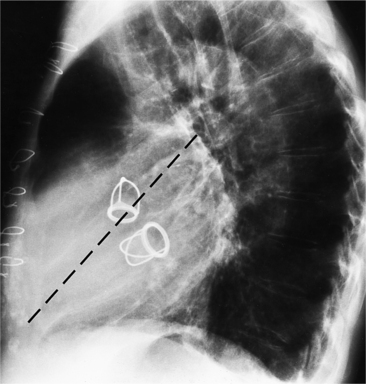
Aortic Valve Calcification
Distinguishing Characteristics
Mitral Annulus Calcification
Distinguishing Patterns
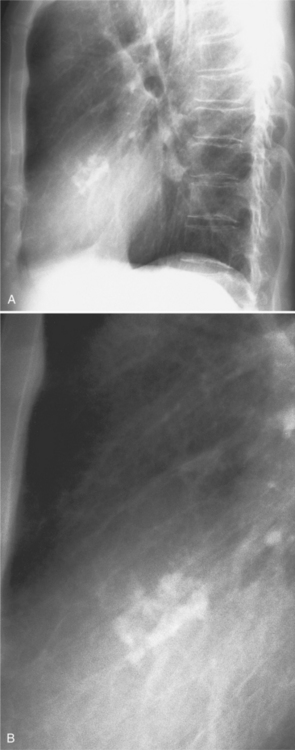
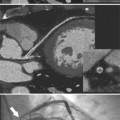
The mitral valve ring may calcify in individuals over age 60. The incidence is four times higher in women. The calcium begins to form in or below the mitral annulus at the junction between the ventricular myocardium and the posterior mitral leaflet. More severe degrees of calcification will form a pattern resembling the letter J, the letter O, or a reversed letter C (Figure 1-19).
Clinical Significance
Aortic stenosis and hypertension have a higher incidence of mitral annulus calcification, possibly because of increased strain exerted on the mitral valve apparatus from the left ventricular pressure overload. For the same reason, the tricuspid annulus rarely may calcify when right ventricular pressures have been chronically increased (Fig. 1-20).